We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox SN2
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
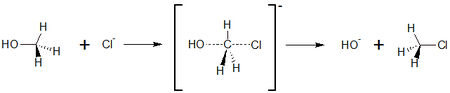
Typically, alkanes with a substituent in primary position undergo S<sub>N</sub>2 reactions. In contrast to a S<sub>N</sub>1 reaction, no stable carbo cation can be formed, therefore, another way is taken. | Typically, alkanes with a substituent in primary position undergo S<sub>N</sub>2 reactions. In contrast to a S<sub>N</sub>1 reaction, no stable carbo cation can be formed, therefore, another way is taken. | ||
The S<sub>N</sub>2 <jmol><jmolLink><script>anim mode once; frame range 1 10; delay 0.5; frame play</script><text>reaction starts</text></jmolLink></jmol> by establishing a so called ''intermediate'' state, with both educts comeing close to each other. By this, the bond of the leaving group is partly broken, and the bond to the new group is partly formed. The formation of this intermediate state is the rate determining step of the reaction. | The S<sub>N</sub>2 <jmol><jmolLink><script>anim mode once; frame range 1 10; delay 0.5; frame play</script><text>reaction starts</text></jmolLink></jmol> by establishing a so called ''intermediate'' state, with both educts comeing close to each other. By this, the bond of the leaving group is partly broken, and the bond to the new group is partly formed. The formation of this intermediate state is the rate determining step of the reaction. | ||
| - | In the <jmol><jmolLink><script>anim mode once; frame range 11 20; delay 0.5; frame play</script><text>second step</text></jmolLink></jmol>, the bond of the leaving group is completely broken, and at the same time the bond to the new substituent is completely formed. | + | In the <jmol><jmolLink><script>anim mode once; frame range 11 20; delay 0.5; frame play</script><text>second step</text></jmolLink></jmol>, the bond of the leaving group is completely broken, and at the same time the bond to the new substituent is completely formed.<br> |
[[Image:reaction_scheme_sn2.jpg|450px]] <br> | [[Image:reaction_scheme_sn2.jpg|450px]] <br> | ||
The S<sub>N</sub>2 reaction has a very interesting stereochemistry inversion of the stereocenter<ref>PMID: 27505286 </ref> with an animated example of the substitution of chloride and methanol shown. Please click on the buttons below to '''animate''' the reaction with different representations. Use the '''popup''' button to enlarge the view and the '''quality''' button to turn on anti-aliasing. | The S<sub>N</sub>2 reaction has a very interesting stereochemistry inversion of the stereocenter<ref>PMID: 27505286 </ref> with an animated example of the substitution of chloride and methanol shown. Please click on the buttons below to '''animate''' the reaction with different representations. Use the '''popup''' button to enlarge the view and the '''quality''' button to turn on anti-aliasing. | ||
Revision as of 18:40, 18 July 2021
SN2-substitution of chloride and methanol
| |||||||||||
See also
SN1 reaction: Substitution of Cl− and tert-Butanol
References
- ↑ Wang Y, Song H, Szabo I, Czako G, Guo H, Yang M. Mode-Specific SN2 Reaction Dynamics. J Phys Chem Lett. 2016 Sep 1;7(17):3322-7. doi: 10.1021/acs.jpclett.6b01457. Epub, 2016 Aug 12. PMID:27505286 doi:http://dx.doi.org/10.1021/acs.jpclett.6b01457
- ↑ ChiLe Web Site