We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox SN2
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
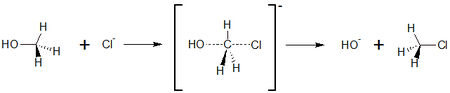
==S<sub>N</sub>2-substitution of chloride and methanol== | ==S<sub>N</sub>2-substitution of chloride and methanol== | ||
| - | <StructureSection load='' size='350' side='right' caption='SN2 - Substitution of Cl and Methanol' scene='88/887016/Sn2_cl_rt/ | + | <StructureSection load='' size='350' side='right' caption='SN2 - Substitution of Cl and Methanol' scene='88/887016/Sn2_cl_rt/6'> |
SN2 reaction is a basic reaction type in organic chemistry. The letter S<sub>N</sub> stands for nulceophilic Substitution, the number 2 stands for bimolecular, with both reactions partners are involved in the reaction rate-determining step. It also exists an S<sub>N</sub>1 reaction; here, only one reaction partner is involved in this step. On the other side, SN2 reactions are characterized by exchanging substituents. The substituent that leaves the molecule is called leaving group. | SN2 reaction is a basic reaction type in organic chemistry. The letter S<sub>N</sub> stands for nulceophilic Substitution, the number 2 stands for bimolecular, with both reactions partners are involved in the reaction rate-determining step. It also exists an S<sub>N</sub>1 reaction; here, only one reaction partner is involved in this step. On the other side, SN2 reactions are characterized by exchanging substituents. The substituent that leaves the molecule is called leaving group. | ||
Revision as of 18:49, 18 July 2021
SN2-substitution of chloride and methanol
| |||||||||||
See also
SN1 reaction: Substitution of Cl− and tert-Butanol
References
- ↑ Wang Y, Song H, Szabo I, Czako G, Guo H, Yang M. Mode-Specific SN2 Reaction Dynamics. J Phys Chem Lett. 2016 Sep 1;7(17):3322-7. doi: 10.1021/acs.jpclett.6b01457. Epub, 2016 Aug 12. PMID:27505286 doi:http://dx.doi.org/10.1021/acs.jpclett.6b01457
- ↑ ChiLe Web Site