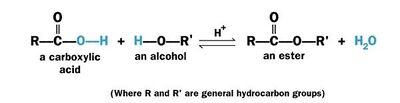
Esterification
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
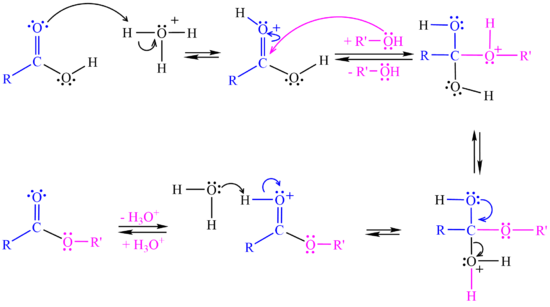
In the <jmol><jmolLink><script>anim mode once; frame range 20 41; delay 0.5; frame play</script><text>third step</text></jmolLink></jmol> a proton is lost at one oxygen atom and bonds to another oxygen atom. | In the <jmol><jmolLink><script>anim mode once; frame range 20 41; delay 0.5; frame play</script><text>third step</text></jmolLink></jmol> a proton is lost at one oxygen atom and bonds to another oxygen atom. | ||
In the <jmol><jmolLink><script>anim mode once; frame range 41 50; delay 0.5; frame play</script><text>fourth step</text></jmolLink></jmol> a water molecule leaves the structure. | In the <jmol><jmolLink><script>anim mode once; frame range 41 50; delay 0.5; frame play</script><text>fourth step</text></jmolLink></jmol> a water molecule leaves the structure. | ||
| - | In the <jmol><jmolLink><script>anim mode once; frame range 51 59; delay 0.5; frame play</script><text>fifth step</text></jmolLink></jmol> a proton (H+) transfers to a base and ester is formed. | + | In the <jmol><jmolLink><script>anim mode once; frame range 51 59; delay 0.5; frame play</script><text>fifth step</text></jmolLink></jmol> a proton (H+) leaves the carbonyl group, transfers to a base and '''ester''' is formed. |
[[Image:Esterification Mechanism.png|550px]] <br> | [[Image:Esterification Mechanism.png|550px]] <br> | ||
Current revision
| |||||||||||
See also
SN1 reaction: Substitution of Cl− and tert-Butanol
SN2 reaction: substitution of Cl− and methanol