Cytochromes
Cytochrome b
Cytochrome b5
Cytochrome b5 (CB) functions as an electron transport carrier for several membrane-bound oxygenases. CB is heme-containing protein. The microsomal and mitochondrial CB are membrane-bound while bacterial and other animal tissue CB are soluble. Cytochrome b562 is the the b-type cytochrome from E. coli.[1] (PDB entry 1b5m[2]) is shown.
Cytochrome bc1 complex
Cytochrome bc1 (Cbc1) functions as the central pump which transfers protons across the cell membrane. The protons are used to power the rotation of ATP synthase. Cbc1 binds ubiquinol which carries hydrogen atoms. Cbc1 separates the protons and the electrons. The protons are released in the inner side of the membrane for use by ATP synthase and the electrons are transferred to cytochrome c or to the outer side of the membrane. Plants use cytochrome b6f in the same manner binding plastoquinol as a hydrogen carrier. Stigmatellin inhibits the Cbc1 electron transfer by binding to its quinone oxidation site. Antimycin inhibits Cbc1 by binding to its quinone reduction site.[3]
More details in Complex_III_of_Electron_Transport_Chain.
Structural highlights
Cbc1 is a composed of 11 proteins and cofactors which include heme-carrying proteins like and and iron-sulfur cluster proteins like . The iron containing moieties are , (where vinyl side chain of heme are replaced by thioether) and . [4]
Cytochrome c
Structural and kinetic studies of imidazole binding to two members of the cytochrome c6 family reveal an important role for a conserved heme pocket residue[5]
is a member of the class I family of c-type cytochromes with a distinctive and a . They function in the photosynthetic electron transport chain of cyanobacteria where they shuttle an electron from the cytochrome b6f complex to photosystem I. Structures of numerous cytochrome c6 proteins have been determined and all have the . In the present work we have solved the structure of the Q51V site-directed variant of Phormidium laminosum cytochrome c6. This project is part of a study that is aimed at gaining insight into protein factors which modulate the heme mid-point redox potential in the cytochrome c6 family. The Q51V variant has been shown to tune over 100 mV of heme redox potential, which for a single heme pocket mutation is very significant and has consequences for function.
The Q51V structure confirms that the has the same side-chain orientation in the heme pocket as found in other cytochrome c6 proteins, that naturally have a Val at this position. The significance of this structure is that the and an . Two other structures of imidazole cyt c-adducts have been reported, but neither appear to undergo the . Both protein and heme structural changes are observed, with the later centered on a accompanied by the and the .
Protein (un)folding studies on cytochrome c have revealed that (un)folding involves structural units called 'foldons'. The regions in the Q51V imidazole-adduct where structural changes occur map well to the two foldons predicted to unfold first in cytochrome c. Thus , leading to the formation of an early unfolding intermediate that is stabilised by , enabling it to be captured in the crystalline form.
Structural model of the [Fe]-hydrogenase/cytochrome C553 complex combining NMR and soft-docking[6]
The shows the specific interaction of the hydrogenase (light blue) with the cytochrome (pink), revealing the path of electron transport from the , through three iron-sulfur clusters, and ending in the cytochrome heme (colored red). Two , CYS 38 in the hydrogenase and CYS10 in the cytochrome, are thought to provide the electron transfer pathway between the two proteins (these scenes were created by Jaime Prilusky, David S. Goodsell, and Eran Hodis).
Conformational control of the binding of diatomic gases to cytochrome c’ [7]
The cytochromes c′ (CYTcp) are found in denitrifying, methanotrophic and photosynthetic bacteria. These proteins are able to form stable adducts with CO and NO but not with O2. The binding of NO to CYTcp currently provides the best structural model for the NO activation mechanism of soluble guanylate cyclase. Ligand binding in CYTcps has been shown to be highly dependent on residues in both the proximal and distal heme pockets. Group 1 CYTcps typically have a phenylalanine residue positioned close to the distal face of heme, while for group 2, this residue is typically leucine. We have structurally, spectroscopically and kinetically characterised the CYTcp from Shewanella frigidimarina , a protein that has a distal phenylalanine residue and a lysine in the proximal pocket in place of the more common arginine (monomer A is colored in red, monomer B in green, and heme group in yellow). in a similar manner to CYTcps previously characterised.
SFCP exhibits biphasic binding kinetics for both NO and CO as a result of the high level of steric hindrance from the aromatic side chain of residue Phe 16. The binding of distal ligands is thus controlled by the conformation of the phenylalanine ring.
- of SFCP (in green;4ulv), RCCP (R. capsulatus; in magenta; 1cpq), RSCP (R. sphaeroides; in red; 1gqa) and RGCP (R. gelatinosus; in cya); 2j8w).
- .
Only a proximal 5-coordinate NO adduct, confirmed by structural data, is observed with no detectable hexacoordinate distal NO adduct.
Rhodothermus marinus cytochrome c
Structure
All members in the C-type cytochrome superfamily contain a heme prosthetic group that is covalently attached to the protein via two thioether bonds to cysteine residues. Most cytochromes c occur in a where the histidine residue is one of the two axial ligands of the heme iron.[8] In monoheme cytochromes c, the other axial position may be left vacant or be occupied by histidine or methionine residues; however, it can sometimes be occupied by cysteine or lysine residues.[8]. In Rmcytc, XX represents a threonine (Thr46) and an alanine residue (Ala47) that help form the loop 2 structure.
The typical monoheme cyt c fold is formed by helices . Rmcytc contains seven α-helices that are folded around the heme, all connected by random coils.[8] The heme group is axially coordinated by , and the disulfide linkages exist at . The heme group in Rmcytc is almost completely shielded from solvent due to it being in a mostly hydrophobic pocket. This pocket is formed in part by the seven helices surrounding the ring, but also by two structures that are uncommon in other cytochromes c. First, a 21 amino acid extension of the N-terminal exists, forming , which wraps around the back of the polypeptide.[8] An extension resembling such has only been seen in Thermus thermophilus; however, the extension occurs at the C-terminus rather than the N-terminus.[9] A second rarity is that of , inserted between helix D and loop 3, that shields the bottom part of the heme from any solvent.[8] In cytochrome c2 as well as mitochondrial cyt c, a similar yet shorter helix was found, though this helix was present at a different place in the primary sequence. Also, instead of helix B', T. thermophilus contains a two-stranded β-sheet.[8] One final note is the number of residues that Rmcytc contains. In general, cyt c contains about two methionines whereas Rmcytc contains seven, located on the left of the heme.[8]
As determined by X-ray crystallography, the Rmcytc structure was found to contain a sulfate ion coordinated to Glu122 via hydrogen bonding to the protonated carboxylate oxygen. In the protein complex, this ion has been seen to mediate crystal contact between neighbouring protein molecules.[8]
The observation of these structural motifs in other C-type cytochromes can support the divergent evolution of cytochromes c.[8] These motifs are present in a number of different bacteria and are seen in similar regions of the secondary structure; however, they exist in the primary sequence in places distinct to the phylum. For example, monoheme cytochromes c in the rest of the Bacteroidetes phylum have an N-terminus extension that is highly conserved to that of Rmcytc, and the regions in the primary structure that correspond to these secondary motifs are not observed in other bacterial phyla.[8] Also, due to these motifs being absent from other phyla, the Bacteroidetes monoheme cyt c has been said to form a new subfamily of cyt c.
Function
Monoheme cytochromes c are involved in electron transport chains in both prokaryotes and eukaryotic mitochondria.[8] They mediate the transfer of electrons mainly from the bc1 complexes or their analogs to heme-copper oxygen reductases (HCOs) in the electron transport chain of oxidative phosphorylation. Heme c containing domains are often found fused to other protein domains such as these HCOs, including the caa3 oxygen reductases[8][10]; these enzymes are membrane-bound and catalyze the reduction of O2 to water.[11] In addition to being involved in oxidative phosphorylation, monoheme cyt c has also been seen to participate in the electron transport chain of photosynthesis.[8] Cytochrome c has also been determined to be a major signalling molecule in the apoptotic pathways.
Electron transport chain
In the electron transport chain (ETC), cyt c shuttles electrons between the respiratory complexes III and IV; complex III is the cytochrome bc1 complex and IV is cyt c oxidase. Initially, the heme iron in cyt c is in the reduced, Fe3+ state; this allows for the uptake of one electron, oxidizing the iron to the Fe2+ state.[12] The ETC in eukaryotes is quite simple compared to that of prokaryotes.
In prokaryotic systems, electrons can enter the ETC at a number of places and multiple donors can be in play; however, the underlying transport system remains the same. Electrons are ultimately transferred from donor to various redox complexes including the bc1 complex and cytochrome c, and finally to a terminal electron acceptor such as molecular oxygen in eukaryotes.[12]
The cytochrome oxidase reaction accounts for nearly 90% of all oxygen uptake in most cells.[12] Due to the large role of cytochromes within the ETC, it would be highly detrimental to the cell if any inhibitors were to be present in the organism. Cyanide and azide bind tightly to the cytochrome oxidase complex, halting electron transport and reducing the overall ATP production.[12]
Apoptosis
In all organisms, cells undergo apoptosis, or programmed cell death, by which there is an extrinsic and an intrinsic pathway. The extrinsic pathway involves an immune response by killer lymphocytes, and once the lymphocyte has been bound to the target cell, an apoptotic cascade occurs.[12] The intrinsic pathway includes cyt c, present in the intermembrane space of mitochondria. In this pathway, the presence of an apoptotic stimulus causes cyt c to be released into the cytosol. Cytochrome c in the cytosol now can be recognized and bound to various apoptotic factors, activating them and forming the apoptosome. The apoptosome recruits caspases, which are activated and result in a caspase cascade to proceed with apoptosis.[12]
Cytochrome c is required for the intrinsic apoptotic process to function properly. Such as with the electron transport chain, a mutation affecting cyt c or other structures in apoptosis could cause either an increase or a decrease in the rate of apoptosis.
For details on decaheme cyt see MtrF
Cytochrome f
Cytochrome f (Cytf) is the largest subunit of the cytochrome b6f complex. This complex transfers electrons from plastocyanin to the two reaction center complexes of oxygenic photosynthetic membranes.[13]
The cytochrome b6f complex contains 4 subunits: Cytf, Cytb6, Rieske iron-sulfur protein and subunit IV. Cytf has an internal chain of water molecules conserved in all its 3D structures. The water chain is assumed to be a proton wire.
in Turnip cytochrome f
(PDB entry 1ctm[14]).
Cytochrome P450
Cytochrome P450 (P450) catalyzes the oxidation of organic substances like lipids. The P450 contains a heme cofactor. The protein is numbered by its gene.[15].
- P450cam, also known as camphor 5-monooxygenase, catalyzes the hydroxylation of camphor.
- P450nor is a Nitric Oxide Reductase P450.
- P450epoK converts epothilone D to epothilone B.
- P450cin monooxygenates 1,8-cineole which is similar to camphor.
- Bifunctional P450/NADPH P450 reductase (P450 BM3) is a fatty acid monooxygenase.
- P450 3A4 see CYP3A4.
- P450 19 family see Aromatase.
Additional details on cytochrome P450 interactions with drugs see
The . The .[16]
Cytochrome c Nitrite Reductase
Shewanella oneidensis Cytochrome c Nitrite Reductase
Laue Crystal Structure of Shewanella oneidensis Cytochrome c Nitrite Reductase from a High-yield Expression System[17]
Cytochrome c nitrite reductase (ccNIR) is a central enzyme of the nitrogen cycle. It binds nitrite, and reduces it by transferring 6 electrons to form ammonia. This ammonia can then be utilized to synthesize nitrogen containing molecules such as amino acids or nucleic acids. However, ccNiR’s primary role is to help extract energy from the reduction; ammonia is simply a potentially useful byproduct. In general, heterotrophic organisms feed on electron-rich substances such as sugars or fatty acids. During the metabolism of these substances large numbers of electrons are produced. Many organisms use oxygen as the final acceptor of these electrons, in which case water is formed. However, some organisms can use alternative electron acceptors such as nitrite, which is where ccNiR comes in.
The ccNiR described here is produced by the Shewanella oneidensis bacterium, which is remarkable in its own right due to the large number of electron acceptors that it can utilize. Shewanella is a facultative anaerobe, which means that it will use oxygen if available, but in the absence of oxygen can get rid of its electrons by dumping them on a wide range of alternate acceptors, of which nitrite is only one example. To handle the electron flow Shewanella uses a large number of promiscuous containing electron transfer proteins. Indeed, Shewanella is exceptionally adept at producing c-heme proteins under fast-growth conditions, which many bacteria commonly used for large-scale laboratory gene expression, such as E. coli, are incapable of unless they are first extensively reprogrammed genetically. Since Shewanella can be easily grown in the lab, and can naturally and easily produce c-hemes, it is an ideal host for generating large quantities of c-heme proteins such as ccNiR.
The 2012 paper by Youngblut et al. [17] describes a genetically modified Shewanella strain that can produce 20 – 40 times more ccNiR per liter of culture than the wild type bacterium. The ccNir so produced can be purified easily and in large amounts. This result is important because c-heme proteins have historically proved difficult to over-express in traditional vectors such as E. coli. With large quantities of Shewanella ccNIR available, Youngblut et al [17] were able to obtain the crystal structure (3ubr) and do a variety of experiments. The ccNIR consists of (colored in darkmagenta and in green) with . In the oxidized ccNIR all central heme irons are Fe3+. They can be subsequently reduced to Fe2+ either by reducing agents or electrochemically. An important conclusion of the paper is that electrons added to ccNiR are likely , rather than localized on individual hemes.
The hemes 3-5 (colored in yellow) and the hemes-2 (colored in seagreen) are six coordinate and used for electron transport only, whereas the two hemes-1 (colored in magenta) are the active sites. Electrons are believed to enter via the hemes-2, but can move between subunits. Though the physiological significance of this result is not yet known, it is possible that delocalizing the electrons keeps the active site redox-potential sufficiently high until enough electrons are accumulated that the reaction with nitrite can take place. That is, CcNIR acts like a capacitor that can store electrons until they are needed. The X-ray structure of the ccNIR reveals the architecture of this capacitor. To solve the structure a non-standard method, the Laue method, was used. This became necessary since attempts to collect a high resolution data set with monochromatic X-ray radiation were not successful. At room temperature the ccNIR crystals are susceptible to radiation damage. Freezing damaged the crystals because a suitable cryoprotectant could not be found. Single pulsed Laue crystallography with 100 ps highly intense polychromatic X-ray pulses provided a solution. A dataset was collected in a few minutes. The crystals were cooled slightly to 0 °C but not frozen. Crystal settings spanned a range of 180 °C and the crystals were orthorhombic. Therefore, a Laue dataset with very high multiplicity and good quality in terms of resolution and Rmerge could be collected. The structure of this ccNIR was then solved by molecular replacement using the E. coli ccNIR as a template. of the S. oneidensis hemes within one monomer with the corresponding E. coli hemes reveals significant similarity. S. oneidensis hemes 3-5, hemes-2, and hemes-1 are colored in yellow, seagreen, and magenta, respectively, whereas their corresponding E. coli hemes are in similar, but darker colors. The of S. oneidensis ccNiR also is similar to that of E. coli ccNiR, except in the region where the enzyme interacts with its physiological electron donor (CymA in the case of S. oneidensis ccNiR, NrfB in the case of the E. coli protein) near heme 2. Subunits of S. oneidensis ccNiR colored in darkmagenta and in green; subunits of E. coli ccNiR colored in hot pink and in deep-sky-blue.
Protein film voltammetry (PFV) experiments performed on S. oneidensis ccNiR films in the absence of substrate produced a broad envelope of reversible signals that span approximately 450 mV. At high pH values the envelope appears as a single peak, whereas at pH values below 7 the envelope appears to be composed of two large overlapping peaks. At pH values below 6 and at 0 °C, the envelope of signal can be better resolved and more than two peaks can be observed. This resulting envelope of signal can be deconvoluted as the sum of five one-electron peaks, each corresponding to one of the five hemes in a ccNiR monomer (see image below).
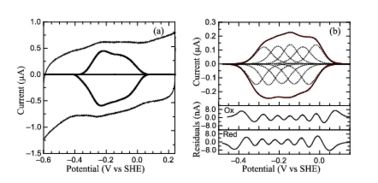
PFV of
S. oneidensis ccNiR (a) Typical signal on a graphite electrode. (b) Baselinesubtracted non-turnover voltammogram
The Ca2+ ion within is coordinated in bidentate fashion by , and in monodentate fashion by the backbone carbonyls, and the side-chain carbonyl. In the S. oneidensis structure only is assigned to the Ca2+ ion in subunit B. In subunit A the difference electron density that represents this water molecule is very close to the noise level, and it is difficult to identify even one water molecule there. The come near to the open calcium coordination sites, but are not within bonding distance. Instead they interact with the water molecule that is weakly coordinated to the Ca2+ ion. The ccNiR calcium ions appear to play a vital role in organizing the (as was mentioned above hemes-1 are the active sites).
Cytochrome c oxidase
Cytochrome c oxidase (CcO) is a transmembrane protein complex found in bacteria and mitochondria. CcO is the last enzyme in the electron transport chain. [18]
Disease
CcO deficiency is a genetic condition which affects muscle weakness, muscle tone upto severe brain dysfunction in severe cases.
Structural highlights
Mammalian CcO is composed of , , and cytochrome a and a3. The fully oxidized form of molecule. . [19] Bacterial CcO is composed of 2 subunits.
Cytochrome c peroxidase
Cytochrome c peroxidase (CcP) is a protoporphyrin-containing enzyme which reduces hydrogen peroxide to water.[20]
Structural highlights
The (PDB entry 1dcc).[21] Water molecules are shown as red spheres.
Cytochrome P450 hydroxylase
Cytochrome P450 hydroxylase (CPH) acts in the in-chain hydroxylation of lauric acid which is required for the development of the male organ in higher plants[22]. CPH hydroxylyzes the anti-cholesterol natural product herbosidiene[23].
- .
- .
- .
- in Cytochrome P450 hydroxylase from Steptomyces avermitilis (PDB code 5cwe).
Ascorbate peroxidase
Function
Ascorbate peroxidase (APX) catalyzes the conversion of ascorbate and hydrogen peroxide to dehydroascorbate and water thus detoxifying hydrogen peroxide. APX is involved in the glutathione-ascorbate cycle. APX is accumulated in plants in response to heat and drought stress. APX contains a heme group.
Relevance
APX is part of plants antioxidant defense. APX converts H2O2 to water using ascorbate as an electron donor. APX is used in electron microscopy as a genetic tag which can be stained independently of light activation.
Structural highlights
The of APX contains a and confers stability to the Fe state in the heme. [24]

