Growth factors
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
*[[Autocrine motility factor]] | *[[Autocrine motility factor]] | ||
Rabbit <scene name='48/481605/Cv/6'>Phosphoglucose isomerase (autocrine motility factor) active site containing the substrate D-fructose 6-phosphate shows the substrate interacting mainly with residues of one subunit</scene><ref>PMID:11425306</ref>. Water molecules are shown as red spheres. | Rabbit <scene name='48/481605/Cv/6'>Phosphoglucose isomerase (autocrine motility factor) active site containing the substrate D-fructose 6-phosphate shows the substrate interacting mainly with residues of one subunit</scene><ref>PMID:11425306</ref>. Water molecules are shown as red spheres. | ||
| - | *[[Bone morphogenetic protein]]. For details on BMP-7 see [[Student Projects for UMass Chemistry 423 Spring 2012-4]]. | + | *[[Bone morphogenetic protein]]. For details on BMP-7 see [[Student Projects for UMass Chemistry 423 Spring 2012-4]]. See also [[Growth differentiation factor]]. |
*[[Colony-stimulating factor]] and [[Colony-stimulating factor receptor]] | *[[Colony-stimulating factor]] and [[Colony-stimulating factor receptor]] | ||
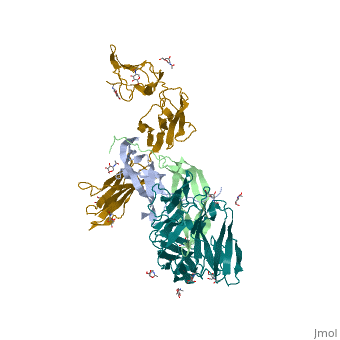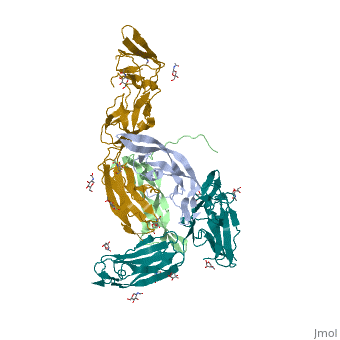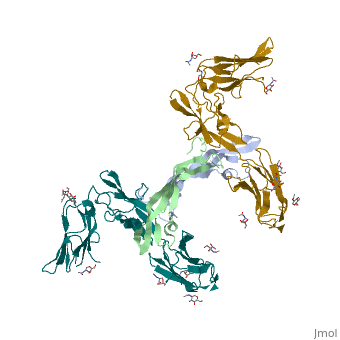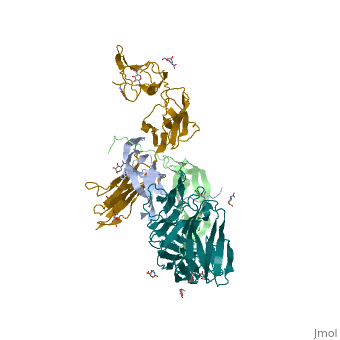
The structure of the complex between M-CSF and its receptor shows that a <scene name='80/801266/Cv/3'>dimer of M-CSF binds to 2 molecules of the receptor</scene>. There are <scene name='80/801266/Cv/5'>two binding sites between the molecules designated site I and site II</scene><ref>PMID:26235028</ref>. <scene name='80/801266/Cv/4'>All interactions</scene>. | The structure of the complex between M-CSF and its receptor shows that a <scene name='80/801266/Cv/3'>dimer of M-CSF binds to 2 molecules of the receptor</scene>. There are <scene name='80/801266/Cv/5'>two binding sites between the molecules designated site I and site II</scene><ref>PMID:26235028</ref>. <scene name='80/801266/Cv/4'>All interactions</scene>. | ||
| Line 45: | Line 45: | ||
FGFR consist of an extracellular ligand-binding domain (LBD), transmembrane helix domain and cytoplasmic tyrosine kinase activity domain (TKD) with phosphorylated tyrosine designated PTR. FGFR LBD contains 3 immunoglobulin-like domains D1, D2 and D3. <scene name='54/544712/Cv/2'>Human fibroblast growth factor receptor 1 ligand-binding domain modules D2 and D3 with 2 molecules of fibroblast growth factor 1</scene> (PDB code [[1evt]]). | FGFR consist of an extracellular ligand-binding domain (LBD), transmembrane helix domain and cytoplasmic tyrosine kinase activity domain (TKD) with phosphorylated tyrosine designated PTR. FGFR LBD contains 3 immunoglobulin-like domains D1, D2 and D3. <scene name='54/544712/Cv/2'>Human fibroblast growth factor receptor 1 ligand-binding domain modules D2 and D3 with 2 molecules of fibroblast growth factor 1</scene> (PDB code [[1evt]]). | ||
*[[Growth differentiation factor]] | *[[Growth differentiation factor]] | ||
| - | For more details see [[Group:MUZIC:Myostatin]]. | + | For more details see [[Group:MUZIC:Myostatin]]. See also [[Bone morphogenetic protein]]. |
*[[Hepatocyte growth factor]] and [[Hepatocyte growth factor receptor]] | *[[Hepatocyte growth factor]] and [[Hepatocyte growth factor receptor]] | ||
*[[Insulin]] and [[Insulin receptor]] | *[[Insulin]] and [[Insulin receptor]] | ||
Revision as of 13:44, 1 August 2021
| |||||||||||
References
- ↑ Mohedas AH, Wang Y, Sanvitale CE, Canning P, Choi S, Xing X, Bullock AN, Cuny GD, Yu PB. Structure-activity relationship of 3,5-diaryl-2-aminopyridine ALK2 inhibitors reveals unaltered binding affinity for fibrodysplasia ossificans progressiva causing mutants. J Med Chem. 2014 Oct 9;57(19):7900-15. doi: 10.1021/jm501177w. Epub 2014 Sep 4. PMID:25101911 doi:http://dx.doi.org/10.1021/jm501177w
- ↑ Lee JH, Chang KZ, Patel V, Jeffery CJ. Crystal structure of rabbit phosphoglucose isomerase complexed with its substrate D-fructose 6-phosphate. Biochemistry. 2001 Jul 3;40(26):7799-805. PMID:11425306
- ↑ Felix J, De Munck S, Verstraete K, Meuris L, Callewaert N, Elegheert J, Savvides SN. Structure and Assembly Mechanism of the Signaling Complex Mediated by Human CSF-1. Structure. 2015 Jul 21. pii: S0969-2126(15)00272-5. doi:, 10.1016/j.str.2015.06.019. PMID:26235028 doi:http://dx.doi.org/10.1016/j.str.2015.06.019
- ↑ Zhang C, Ibrahim PN, Zhang J, Burton EA, Habets G, Zhang Y, Powell B, West BL, Matusow B, Tsang G, Shellooe R, Carias H, Nguyen H, Marimuthu A, Zhang KY, Oh A, Bremer R, Hurt CR, Artis DR, Wu G, Nespi M, Spevak W, Lin P, Nolop K, Hirth P, Tesch GH, Bollag G. Design and pharmacology of a highly specific dual FMS and KIT kinase inhibitor. Proc Natl Acad Sci U S A. 2013 Mar 14. PMID:23493555 doi:http://dx.doi.org/10.1073/pnas.1219457110
- ↑ Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007 May;17(5):230-8. Epub 2007 Apr 8. PMID:17420126 doi:http://dx.doi.org/10.1016/j.tcb.2007.03.004
- ↑ Himanen JP, Yermekbayeva L, Janes PW, Walker JR, Xu K, Atapattu L, Rajashankar KR, Mensinga A, Lackmann M, Nikolov DB, Dhe-Paganon S. Architecture of Eph receptor clusters. Proc Natl Acad Sci U S A. 2010 May 26. PMID:20505120
- ↑ Davis TL, Walker JR, Allali-Hassani A, Parker SA, Turk BE, Dhe-Paganon S. Structural recognition of an optimized substrate for the ephrin family of receptor tyrosine kinases. FEBS J. 2009 Aug;276(16):4395-404. PMID:19678838 doi:http://dx.doi.org/10.1111/j.1742-4658.2009.07147.x
- ↑ Himanen JP, Yermekbayeva L, Janes PW, Walker JR, Xu K, Atapattu L, Rajashankar KR, Mensinga A, Lackmann M, Nikolov DB, Dhe-Paganon S. Architecture of Eph receptor clusters. Proc Natl Acad Sci U S A. 2010 May 26. PMID:20505120
- ↑ Syed RS, Reid SW, Li C, Cheetham JC, Aoki KH, Liu B, Zhan H, Osslund TD, Chirino AJ, Zhang J, Finer-Moore J, Elliott S, Sitney K, Katz BA, Matthews DJ, Wendoloski JJ, Egrie J, Stroud RM. Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature. 1998 Oct 1;395(6701):511-6. PMID:9774108 doi:http://dx.doi.org/10.1038/26773
- ↑ Syed RS, Reid SW, Li C, Cheetham JC, Aoki KH, Liu B, Zhan H, Osslund TD, Chirino AJ, Zhang J, Finer-Moore J, Elliott S, Sitney K, Katz BA, Matthews DJ, Wendoloski JJ, Egrie J, Stroud RM. Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature. 1998 Oct 1;395(6701):511-6. PMID:9774108 doi:http://dx.doi.org/10.1038/26773
- ↑ Kulahin N, Kiselyov V, Kochoyan A, Kristensen O, Kastrup JS, Berezin V, Bock E, Gajhede M. Dimerization effect of sucrose octasulfate on rat FGF1. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008 Jun 1;64(Pt, 6):448-52. Epub 2008 May 16. PMID:18540049 doi:10.1107/S174430910801066X