Beta sheet
From Proteopedia
(→Structure, hydrogen bonding and composition) |
|||
| Line 4: | Line 4: | ||
==Structure, hydrogen bonding and composition== | ==Structure, hydrogen bonding and composition== | ||
<StructureSection load='' size='350' side='right' caption='' scene='88/889825/Toxin_1f94/1'> | <StructureSection load='' size='350' side='right' caption='' scene='88/889825/Toxin_1f94/1'> | ||
| - | The <scene name='88/889825/Toxin_1f94/1'>initial scene</scene> shows a protein with two sheets, one composed of four strands and the other of two. Beta strands are characterized by the extended conformation of the main chain (with phi and psi angles in the upper left quadrant of the [[Ramachandran plot]]) and hydrogen bonds to the neighboring strands. | + | The <scene name='88/889825/Toxin_1f94/1'>initial scene</scene> shows a protein with two sheets, one composed of four strands and the other of two. Beta strands are characterized by the extended conformation of the main chain (with phi and psi angles in the upper left quadrant of the [[Ramachandran plot]]) and hydrogen bonds to the neighboring strands. The beta sheet is also called beta pleated sheet because the peptide planes follow a zig-zag pattern. |
For a strand in the middle of a sheet (as opposed to on its edge), all main chain hydrogen bond donors (the N-H groups) and acceptors (the C=O groups) are part of inter-strand hydrogen bonds. Strands are either in <scene name='88/889825/6l26_direction/1'>a parallel or an antiparallel arrangement</scene>, resulting in different <scene name='88/889825/6l26_direction/2'>hydrogen bonding patterns</scene> [https://www.ncbi.nlm.nih.gov/books/NBK22580/#_A328_]. Accordingly, beta sheets are classified as parallel, antiparallel or mixed. The antiparallel arrangement of strands is more prevalent <ref>DOI:10.1021/ci200027d</ref>. The beta strands on the edge of a sheet will have hydrogen bond donors and acceptors that have no other strand to partner with unless the sheet forms a cylindrical structure called a beta barrel, such as in the [[green fluorescent protein]] structure. | For a strand in the middle of a sheet (as opposed to on its edge), all main chain hydrogen bond donors (the N-H groups) and acceptors (the C=O groups) are part of inter-strand hydrogen bonds. Strands are either in <scene name='88/889825/6l26_direction/1'>a parallel or an antiparallel arrangement</scene>, resulting in different <scene name='88/889825/6l26_direction/2'>hydrogen bonding patterns</scene> [https://www.ncbi.nlm.nih.gov/books/NBK22580/#_A328_]. Accordingly, beta sheets are classified as parallel, antiparallel or mixed. The antiparallel arrangement of strands is more prevalent <ref>DOI:10.1021/ci200027d</ref>. The beta strands on the edge of a sheet will have hydrogen bond donors and acceptors that have no other strand to partner with unless the sheet forms a cylindrical structure called a beta barrel, such as in the [[green fluorescent protein]] structure. | ||
Revision as of 14:47, 8 August 2021
This page is under construction, --Karsten Theis 09:52, 7 August 2021 (UTC)
A beta sheet is a type of secondary structure, i.e. a description of how the main chain of a protein is arranged in space. It is composed of at least two beta strands. Beta strands have repetitive regular secondary structure (just like the alpha helix), i.e. all residues have similar conformation and hydrogen bonding, and it can be of arbitrary length.
Contents |
Structure, hydrogen bonding and composition
| |||||||||||
Connectedness or topology of beta sheets
In a beta sheet, neighboring strands can either have a parallel or an antiparallel orientation, resulting in different hydrogen bonding pattern. The strands, if part of the same subunit, will all be connected as part of a single polypeptide. The parallel or antiparallel orientations and how strands are connected in their primary sequence is shown in topology ; these are useful to compare different protein folds. One example of such a beta sheet topology is the so-called greek key - an all-antiparallel sheet with four strands.
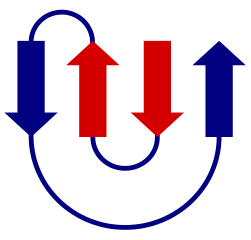
Some topologies occur very often while others have not yet been observed. There are some rules of thumb about preferred topologies. [2]
Types of proteins and folds that contain sheets
Beta sheets in soluble (globular) proteins
Beta sheets in transmembrane proteins
Beta sheets in amyloid fibrils
History
Alpha helices and beta sheets are named after two conformations of keratin, a fiber occuring in mammals (wool, hair, quills) [3]. Alpha keratin is composed of colide coils of alpha helices, whereas hard stretching these fibers in water changes the conformation to beta sheets. The two conformations show different diffraction data under X-ray illumination.
Experimental evidence
Apart from the historical fiber diffraction data, various spectroscopic techniques may be used to show the presence of beta sheets. Circular dichroism (CD) or infrared (IR) spectroscopy allows an estimate of the beta sheet content of a protein sample. NMR spectroscopy, after resonance assignment, allows secondary structure assignment residue by residue based on chemical shifts of the alpha carbon and beta carbon resonances.
Quiz
See Also
Physical model of a beta sheet for teaching
References
- ↑ Tsutsumi M, Otaki JM. Parallel and antiparallel beta-strands differ in amino acid composition and availability of short constituent sequences. J Chem Inf Model. 2011 Jun 27;51(6):1457-64. doi: 10.1021/ci200027d. Epub 2011, May 11. PMID:21520893 doi:http://dx.doi.org/10.1021/ci200027d
- ↑ Minami S, Chikenji G, Ota M. Rules for connectivity of secondary structure elements in protein: Two-layer alphabeta sandwiches. Protein Sci. 2017 Nov;26(11):2257-2267. doi: 10.1002/pro.3285. Epub 2017 Sep 19. PMID:28856751 doi:http://dx.doi.org/10.1002/pro.3285
- ↑ Kreplak L, Doucet J, Dumas P, Briki F. New aspects of the alpha-helix to beta-sheet transition in stretched hard alpha-keratin fibers. Biophys J. 2004 Jul;87(1):640-7. doi: 10.1529/biophysj.103.036749. PMID:15240497 doi:http://dx.doi.org/10.1529/biophysj.103.036749
