Aminoacyl tRNA Synthetase
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
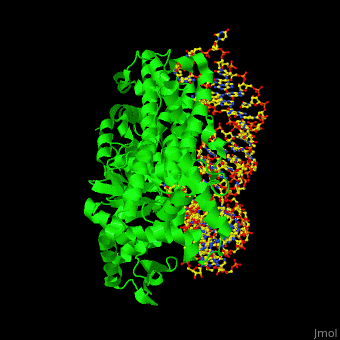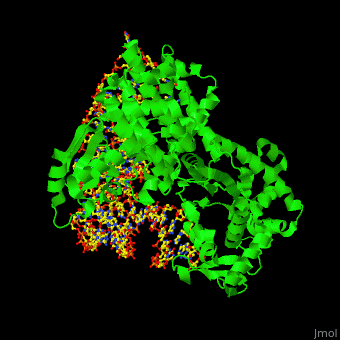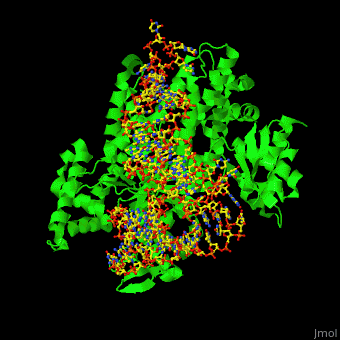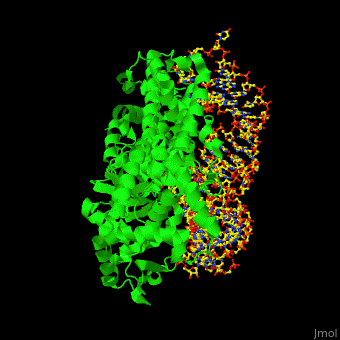
One very interesting question in biology is how does an aminoacyl-tRNA synthetase recognize a particular tRNA and charge it with the correct amino acid? This is a challenging problem, since all tRNAs have the same general structure. Interestingly, different tRNA synthetases accomplish this goal in different ways. | One very interesting question in biology is how does an aminoacyl-tRNA synthetase recognize a particular tRNA and charge it with the correct amino acid? This is a challenging problem, since all tRNAs have the same general structure. Interestingly, different tRNA synthetases accomplish this goal in different ways. | ||
| - | The <scene name='44/444597/Protein_surface_interaction/2'>glutaminyl-tRNA synthetase</scene> (GlnRS) interacts with both <scene name='44/444597/Acceptor_and_anticodon_interac/1'>the anticodon loop and the acceptor stem</scene>. Genetic and biochemical data indicate that GlnRS interacts with all three bases of the <scene name='44/444597/Anticodon_loop/ | + | The <scene name='44/444597/Protein_surface_interaction/2'>glutaminyl-tRNA synthetase</scene> (GlnRS) interacts with both <scene name='44/444597/Acceptor_and_anticodon_interac/1'>the anticodon loop and the acceptor stem</scene>. Genetic and biochemical data indicate that GlnRS interacts with all three bases of the <scene name='44/444597/Anticodon_loop/3'>anticodon loop</scene>, which are unstacked and splay outward so they can bind in separate recognition pockets of GlnRS. The 3' end of tRNAgln plunges deeply into a protein pocket that also binds the enzyme's <scene name='44/444597/Atp_binding_site/1'>ATP</scene> and glutamine substrates. |
</StructureSection> | </StructureSection> | ||
Revision as of 19:43, 21 September 2021
| |||||||||||
References
- ↑ Cavarelli J, Moras D. Recognition of tRNAs by aminoacyl-tRNA synthetases. FASEB J. 1993 Jan;7(1):79-86. PMID:8422978
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Joel L. Sussman, Ann Taylor