We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Ku protein
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
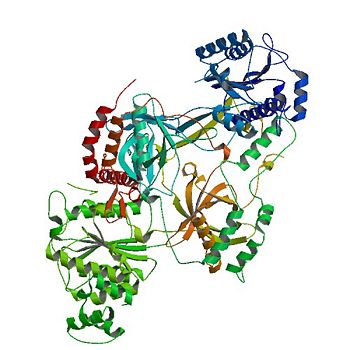
<StructureSection load='1JEY' size='350' side='right' caption='Structure of the Ku heterodimer bound to DNA (PDB entry [[1jey]])' scene='56/567269/Ku_heterodimer/4'> | <StructureSection load='1JEY' size='350' side='right' caption='Structure of the Ku heterodimer bound to DNA (PDB entry [[1jey]])' scene='56/567269/Ku_heterodimer/4'> | ||
== Overview == | == Overview == | ||
| - | The '''Ku protein''' binds to the ends of double-strand breaks and it is required in DNA-repair for non-homologous end joining. The eukaryotic Ku protein is a | + | The '''Ku protein''' or '''X-ray repair cross-complementing protein''' binds to the ends of double-strand breaks and it is required in DNA-repair for non-homologous end joining. The eukaryotic Ku protein is a |
<scene name='56/567269/Ku_heterodimer/3'>heterodimer</scene> | <scene name='56/567269/Ku_heterodimer/3'>heterodimer</scene> | ||
composed of a | composed of a | ||
| Line 32: | Line 32: | ||
In terms of protein structure, the α/β-Domain contributes little to the dimer interface between the subunits. | In terms of protein structure, the α/β-Domain contributes little to the dimer interface between the subunits. | ||
The C terminus of the domain can be bound to other repair molecules, using the α/β-Domain as a scaffold.<ref name="Walker"/> | The C terminus of the domain can be bound to other repair molecules, using the α/β-Domain as a scaffold.<ref name="Walker"/> | ||
| - | |||
=== β-barrel === | === β-barrel === | ||
| Line 38: | Line 37: | ||
The <scene name='56/567269/Ku70_dimer/4'>β-barrel</scene> is the main source of interactions of the Ku heterodimer itself and DNA helix, with each β-barrel being composed of seven β strands with the majority in antiparallel arrangement.<ref name="Walker"/> | The <scene name='56/567269/Ku70_dimer/4'>β-barrel</scene> is the main source of interactions of the Ku heterodimer itself and DNA helix, with each β-barrel being composed of seven β strands with the majority in antiparallel arrangement.<ref name="Walker"/> | ||
The quantity of the strands lends the structures to be symmetrical. Both β-barrel in the dimer form the base of the cradle by fitting in the grooves of DNA. | The quantity of the strands lends the structures to be symmetrical. Both β-barrel in the dimer form the base of the cradle by fitting in the grooves of DNA. | ||
| - | |||
=== C-terminal arm === | === C-terminal arm === | ||
| Line 44: | Line 42: | ||
The <scene name='56/567269/Ku70_dimer/7'>C-terminal arm</scene> is an α-helical domain that associates with the β-barrel of the opposite subunit, with the arm stretching across the DNA helix.<ref name="Walker"/> | The <scene name='56/567269/Ku70_dimer/7'>C-terminal arm</scene> is an α-helical domain that associates with the β-barrel of the opposite subunit, with the arm stretching across the DNA helix.<ref name="Walker"/> | ||
As a result, the C-terminal arm strengthens the cradle composed of the two β-barrels. | As a result, the C-terminal arm strengthens the cradle composed of the two β-barrels. | ||
| - | |||
=== DNA binding ring === | === DNA binding ring === | ||
| Line 51: | Line 48: | ||
By binding DNA, Ku realigns the the strands and protects the molecule from degradation and unwanted bonds while NHEJ occurs.<ref name="Walker"/> | By binding DNA, Ku realigns the the strands and protects the molecule from degradation and unwanted bonds while NHEJ occurs.<ref name="Walker"/> | ||
The regulation of the DNA binding ring of Ku is still under research, with data supporting oxidative stress and redox reactions decreasing the association of the Ku heterodimer with bound DNA through alterations in cysteine residues on the Ku70 subunit. <ref name="source3"/> <ref name="source4"> PMID: 14585978</ref> | The regulation of the DNA binding ring of Ku is still under research, with data supporting oxidative stress and redox reactions decreasing the association of the Ku heterodimer with bound DNA through alterations in cysteine residues on the Ku70 subunit. <ref name="source3"/> <ref name="source4"> PMID: 14585978</ref> | ||
| - | |||
| - | |||
== Function == | == Function == | ||
| Line 64: | Line 59: | ||
Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | [[1jey]] – hKu70 + Ku80 + DNA – human<br /> | + | [[1jey]], [[5y58]] – hKu70 + Ku80 + DNA – human<br /> |
[[1jeq]] – hKu70 + Ku80 <br /> | [[1jeq]] – hKu70 + Ku80 <br /> | ||
| + | [[7axz]] – hKu70 + Ku80 – Cryo EM<br /> | ||
| + | [[6zh6]] – hKu80 + DNA-dependent protein kinase – Cryo EM<br /> | ||
| + | [[7k0y]], [[7k1j]], [[7k1k]], [[7k1n]] – hKu70 + Ku80 + DNA-dependent protein kinase – Cryo EM<br /> | ||
| + | [[6erf]], [[6erg]], [[6erh]] – hKu70 + Ku80 + non-homologous end-joining factor + DNA <br /> | ||
[[1jjr]] – hKu70 C terminal - NMR <br /> | [[1jjr]] – hKu70 C terminal - NMR <br /> | ||
[[1rw2]], [[1q2z]] – hKu80 C terminal - NMR <br /> | [[1rw2]], [[1q2z]] – hKu80 C terminal - NMR <br /> | ||
| - | + | [[6tyt]], [[6tyu]], [[6tyv]], [[6tyw]], [[6tyx]], [[6tyz]] – hKu80 von Willebrand domain 1-242 (mutant) + peptide <br /> | |
| + | [[7lt3]] – hKu70 + Ku80 in NHEJ synaptic complex – Cryo EM<br /> | ||
== References== | == References== | ||
<references /> | <references /> | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Revision as of 07:23, 14 October 2021
| |||||||||||
3D Structures of Ku protein
Updated on 14-October-2021
1jey, 5y58 – hKu70 + Ku80 + DNA – human
1jeq – hKu70 + Ku80
7axz – hKu70 + Ku80 – Cryo EM
6zh6 – hKu80 + DNA-dependent protein kinase – Cryo EM
7k0y, 7k1j, 7k1k, 7k1n – hKu70 + Ku80 + DNA-dependent protein kinase – Cryo EM
6erf, 6erg, 6erh – hKu70 + Ku80 + non-homologous end-joining factor + DNA
1jjr – hKu70 C terminal - NMR
1rw2, 1q2z – hKu80 C terminal - NMR
6tyt, 6tyu, 6tyv, 6tyw, 6tyx, 6tyz – hKu80 von Willebrand domain 1-242 (mutant) + peptide
7lt3 – hKu70 + Ku80 in NHEJ synaptic complex – Cryo EM
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 2001 Aug 9;412(6847):607-14. PMID:11493912 doi:10.1038/35088000
- ↑ Bennett SM, Neher TM, Shatilla A, Turchi JJ. Molecular analysis of Ku redox regulation. BMC Mol Biol. 2009 Aug 28;10:86. doi: 10.1186/1471-2199-10-86. PMID:19715578 doi:http://dx.doi.org/10.1186/1471-2199-10-86
- ↑ 3.0 3.1 3.2 Polotnianka RM, Li J, Lustig AJ. The yeast Ku heterodimer is essential for protection of the telomere against nucleolytic and recombinational activities. Curr Biol. 1998 Jul 2;8(14):831-4. PMID:9663392
- ↑ 4.0 4.1 Bertuch AA, Lundblad V. The Ku heterodimer performs separable activities at double-strand breaks and chromosome termini. Mol Cell Biol. 2003 Nov;23(22):8202-15. PMID:14585978
- ↑ Berg, Jeremy M., John L. Tymoczko, and Lubert Stryer. Biochemistry. 7th ed. New York: W.H. Freeman and, 2012. ISBN-10: 1-4292-2936-5