We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
1dsn
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
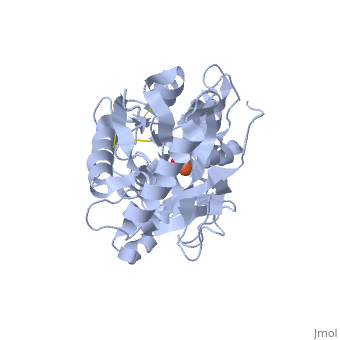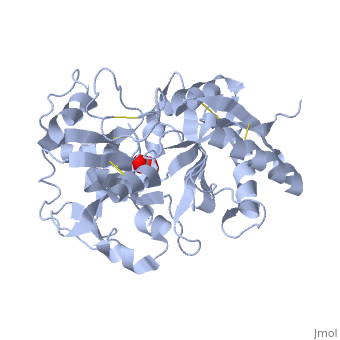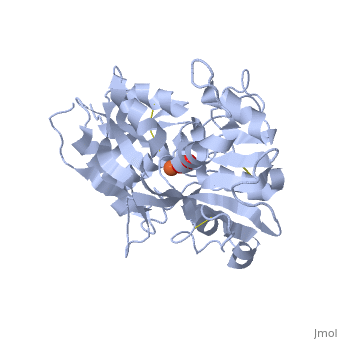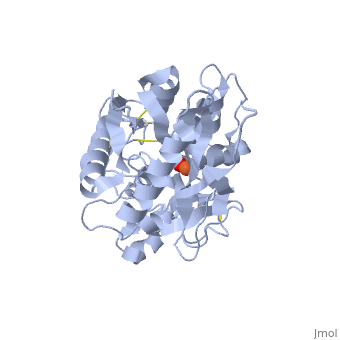
<StructureSection load='1dsn' size='340' side='right'caption='[[1dsn]], [[Resolution|resolution]] 2.05Å' scene=''> | <StructureSection load='1dsn' size='340' side='right'caption='[[1dsn]], [[Resolution|resolution]] 2.05Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
| - | <table><tr><td colspan='2'>[[1dsn]] is a 1 chain structure with sequence from [ | + | <table><tr><td colspan='2'>[[1dsn]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/Human Human]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1DSN OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1DSN FirstGlance]. <br> |
</td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=CO3:CARBONATE+ION'>CO3</scene>, <scene name='pdbligand=FE:FE+(III)+ION'>FE</scene></td></tr> | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=CO3:CARBONATE+ION'>CO3</scene>, <scene name='pdbligand=FE:FE+(III)+ION'>FE</scene></td></tr> | ||
| - | <tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">LFN ([ | + | <tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">LFN ([https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9606 HUMAN])</td></tr> |
| - | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[ | + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1dsn FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1dsn OCA], [https://pdbe.org/1dsn PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1dsn RCSB], [https://www.ebi.ac.uk/pdbsum/1dsn PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1dsn ProSAT]</span></td></tr> |
</table> | </table> | ||
== Function == | == Function == | ||
| - | [[ | + | [[https://www.uniprot.org/uniprot/TRFL_HUMAN TRFL_HUMAN]] Transferrins are iron binding transport proteins which can bind two Fe(3+) ions in association with the binding of an anion, usually bicarbonate.<ref>PMID:12535064</ref> <ref>PMID:22320386</ref> Lactotransferrin has antimicrobial activity which depends on the extracellular cation concentration.<ref>PMID:12535064</ref> <ref>PMID:22320386</ref> Lactoferroxins A, B and C have opioid antagonist activity. Lactoferroxin A shows preference for mu-receptors, while lactoferroxin B and C have somewhat higher degrees of preference for kappa-receptors than for mu-receptors.<ref>PMID:12535064</ref> <ref>PMID:22320386</ref> The lactotransferrin transferrin-like domain 1 functions as a serine protease of the peptidase S60 family that cuts arginine rich regions. This function contributes to the antimicrobial activity.<ref>PMID:12535064</ref> <ref>PMID:22320386</ref> Isoform DeltaLf: transcription factor with antiproliferative properties and inducing cell cycle arrest. Binds to DeltaLf response element found in the SKP1, BAX, DCPS, and SELH promoters.<ref>PMID:12535064</ref> <ref>PMID:22320386</ref> |
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
| Line 31: | Line 31: | ||
==See Also== | ==See Also== | ||
| - | *[[Human lactoferrin|Human lactoferrin]] | ||
*[[Lactoferrin|Lactoferrin]] | *[[Lactoferrin|Lactoferrin]] | ||
== References == | == References == | ||
Revision as of 15:02, 3 November 2021
D60S N-TERMINAL LOBE HUMAN LACTOFERRIN
| |||||||||||