This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Enkephalin
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
Volume 16, Issue 12,1975,Pages 1753-1758,ISSN 0024-3205,https://doi.org/10.1016/0024-3205(75)90268-4.</ref> <ref>Hans W. Kosterlitz, John Hughes, | Volume 16, Issue 12,1975,Pages 1753-1758,ISSN 0024-3205,https://doi.org/10.1016/0024-3205(75)90268-4.</ref> <ref>Hans W. Kosterlitz, John Hughes, | ||
Some thoughts on the significance of enkephalin, the endogenous ligand, Life Sciences, Volume 17, Issue 1, 1975, Pages 91-96, ISSN 0024-3205, https://doi.org/10.1016/0024-3205(75)90243-X.</ref>. They are pentapeptides that can be divided into two groups based on their carboxy-terminal amino acids: '''methionine-enkephalin''' and '''leucine-enkephalin'''. | Some thoughts on the significance of enkephalin, the endogenous ligand, Life Sciences, Volume 17, Issue 1, 1975, Pages 91-96, ISSN 0024-3205, https://doi.org/10.1016/0024-3205(75)90243-X.</ref>. They are pentapeptides that can be divided into two groups based on their carboxy-terminal amino acids: '''methionine-enkephalin''' and '''leucine-enkephalin'''. | ||
| - | Enkephalin acts as a neurotransmitter through opioid receptors, more specifically through the <scene name='89/897677/Leu-enkephalin_bind/1'>classical opioid receptor δ</scene> <ref name="cullen">Cullen JM, Cascella M. Physiology, Enkephalin. [Updated 2021 Mar 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557764/ </ref>. The main functions of enkephalins include analgesia, but they are also involved in the control of respiratory, cardiovascular and gastrointestinal functions, and participate in neuroendocrine regulation <ref>Marcotte, I., Separovic, F., Auger, M., & Gagné, S. M. (2004). A multidimensional 1H NMR investigation of the conformation of methionine-enkephalin in fast-tumbling bicelles. Biophysical journal, 86(3), 1587–1600. https://doi.org/10.1016/S0006-3495(04)74226-5</ref> <ref>Cesselin, F. 1997. Endomorphines, Récepteurs des Opioïdes et Nociception. In Douleurs: Bases Fondamentales, Pharmacologie, Douleurs Aiguës, Douleurs Chroniques, Thérapeutiques. L. Brasseur, M. Chauvin, G. Guilbaud, and P. Guesnon, editors. Maloine, Paris, France.</ref> <ref>Fuxe, K., Borroto-Escuela, D. O., Romero-Fernandez, W., Diaz-Cabiale, Z., Rivera, A., Ferraro, L., Tanganelli, S., Tarakanov, A. O., Garriga, P., Narváez, J. A., Ciruela, F., Guescini, M., & Agnati, L. F. (2012). Extrasynaptic neurotransmission in the modulation of brain function. Focus on the striatal neuronal-glial networks. Frontiers in physiology, 3, 136. https://doi.org/10.3389/fphys.2012.00136</ref>. | + | Enkephalin acts as a neurotransmitter through opioid receptors, more specifically through the <scene name='89/897677/Leu-enkephalin_bind/1'>classical opioid receptor δ</scene> <ref name="cullen">Cullen JM, Cascella M. Physiology, Enkephalin. [Updated 2021 Mar 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557764/ </ref>. The main functions of enkephalins include analgesia, but they are also involved in the control of respiratory, cardiovascular and gastrointestinal functions, and participate in neuroendocrine regulation <ref name="marcotte">Marcotte, I., Separovic, F., Auger, M., & Gagné, S. M. (2004). A multidimensional 1H NMR investigation of the conformation of methionine-enkephalin in fast-tumbling bicelles. Biophysical journal, 86(3), 1587–1600. https://doi.org/10.1016/S0006-3495(04)74226-5</ref> <ref>Cesselin, F. 1997. Endomorphines, Récepteurs des Opioïdes et Nociception. In Douleurs: Bases Fondamentales, Pharmacologie, Douleurs Aiguës, Douleurs Chroniques, Thérapeutiques. L. Brasseur, M. Chauvin, G. Guilbaud, and P. Guesnon, editors. Maloine, Paris, France.</ref> <ref>Fuxe, K., Borroto-Escuela, D. O., Romero-Fernandez, W., Diaz-Cabiale, Z., Rivera, A., Ferraro, L., Tanganelli, S., Tarakanov, A. O., Garriga, P., Narváez, J. A., Ciruela, F., Guescini, M., & Agnati, L. F. (2012). Extrasynaptic neurotransmission in the modulation of brain function. Focus on the striatal neuronal-glial networks. Frontiers in physiology, 3, 136. https://doi.org/10.3389/fphys.2012.00136</ref>. |
Enkephalin is generated from the cleavage of the precursor '''pro-enkephalin''', resulting in Met-enkephalin or Leu-enkephalin. The processing of one molecule of pro-enkephalin generates six copies of <scene name='89/897677/Met-enkephalin/1'>Met-enkephalin</scene> and one copy of Leu-enkephalin <ref name="cullen"/>. | Enkephalin is generated from the cleavage of the precursor '''pro-enkephalin''', resulting in Met-enkephalin or Leu-enkephalin. The processing of one molecule of pro-enkephalin generates six copies of <scene name='89/897677/Met-enkephalin/1'>Met-enkephalin</scene> and one copy of Leu-enkephalin <ref name="cullen"/>. | ||
| Line 14: | Line 14: | ||
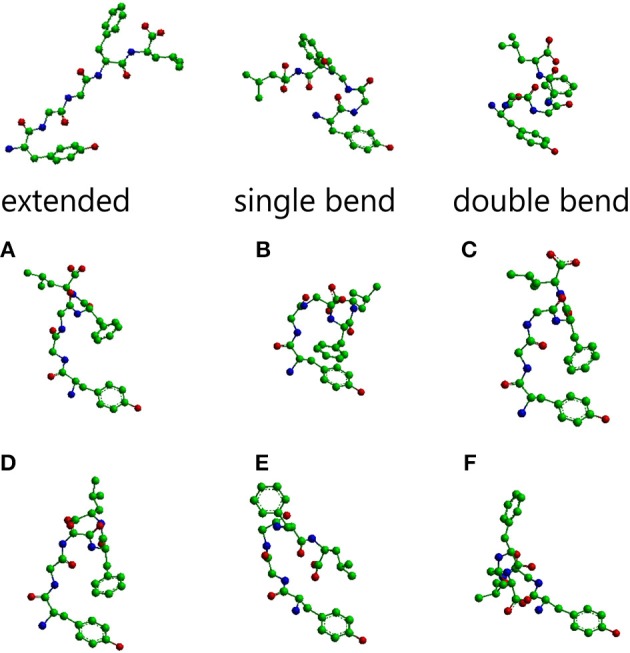
The main conformations of enkephalin found in crystals have been classified in three categories, described as “extended,” “single bend,” and “double bend.”<ref>Deschamps J. R., George C., Flippen-Anderson J. L. (1996). Structural studies of opioid peptides: a review of recent progress in x-ray diffraction studies. Biopolymers 40, 121–139. 10.1002/bip.360400102</ref>. The picture below represents molecular models of Leu-enkephalin in the three main conformations found in solid state determinations when the peptide is dissolved in a DMSO/water cryomixture at 275 K. <ref>Amodeo P., Naider F., Picone D., Tancredi T., Temussi P. A. (1998). Conformational sampling of bioactive conformers: a low temperature NMR study of 15N-Leu-enkephalin. J. Pept. Sci. 4, 253–265.</ref>. | The main conformations of enkephalin found in crystals have been classified in three categories, described as “extended,” “single bend,” and “double bend.”<ref>Deschamps J. R., George C., Flippen-Anderson J. L. (1996). Structural studies of opioid peptides: a review of recent progress in x-ray diffraction studies. Biopolymers 40, 121–139. 10.1002/bip.360400102</ref>. The picture below represents molecular models of Leu-enkephalin in the three main conformations found in solid state determinations when the peptide is dissolved in a DMSO/water cryomixture at 275 K. <ref>Amodeo P., Naider F., Picone D., Tancredi T., Temussi P. A. (1998). Conformational sampling of bioactive conformers: a low temperature NMR study of 15N-Leu-enkephalin. J. Pept. Sci. 4, 253–265.</ref>. | ||
[[Image:Leu-Enkephalin_conformations.jpg]] | [[Image:Leu-Enkephalin_conformations.jpg]] | ||
| + | |||
| + | Met-enkephalin amino acid sequence is Tyr-Gly-Gly-Phe-Met, while leu-enkephalin amino acid sequence is Tyr-Gly-Gly-Phe-Leu. As we can see, <scene name='89/897677/Tyr1_and_phe4/1'>tyrosine and phenylalanine rings</scene> of leu-enkephalin are on opposite sides of the backbone and point in different directions. A similar conformation was found for met-enkephalin in Bic/PG <ref name="marcotte"/>. | ||
| + | |||
| + | Variations in membrane composition seems to have an effect on the conformation adopted by enkephalins <ref name="marcotte"/>. There is common agreement that the orientation of the tyrosine and phenylalanine rings with respect to each other dictates the receptor subtype selectivity <ref name="marcotte"/>. It was originally believed that the ''μ''-selective opiates adopted a folded conformation whereas the δ-opiates preferred an extended form <ref name="marcotte"/> <ref>Hansen, P. E., and B. A. Morgan. 1984. Structure-activity relationships in enkephalin peptides. In Opioid Peptides: Biology, Chemistry, and Genetics, Vol. 6. S. Udenfriend and J. Meienhofer, editors. Academic Press, Orlando, FL.</ref>. However, later other studies suggested a folded conformation with the Tyr and Phe aromatic rings in proximity for the δ-selective opiates, whereas the aromatic rings would point in different directions in the ''μ''-type opiates <ref name="marcotte"/> <ref>Belleney, J., G. Gacel, M. C. Fournié-Zalusky, B. Maigret, and B. P. Roques. 1989. δ opioid receptor selectivity induced by conformational constraints in linear enkephalin-related peptides: 1H 400-MHz NMR study and theoretical calculations. Biochemistry. 28:7392–7400.</ref> <ref>Groth, M., J. Malicka, C. Czaplewski, S. Oldziej, L. Lankiewicz, W. Wiczk, and A. Liwo. 1999. Maximum entropy approach to the determination of solution conformation of flexible polypeptides by global conformational analysis and NMR spectroscopy: application to DNS1-c-[D-A2bu2, Trp4, Leu5]-enkephalin and DNS1-c-[D-A2bu2, Trp4, D-Leu5]enkephalin. J. Biomol. NMR. 15:315–330.</ref> <ref>Hruby, V. J., L.-F. Kao, B. M. Pettitt, and M. Karplus. 1988. The conformational properties of the δ-opioid peptide [D-Pen2,D-Pen5]enkephalin in aqueous solution determined by NMR and energy minimization calculations. J. Am. Chem. Soc. 110:3351–3359.</ref> <ref>Keys, C., P. Payne, P. Amsterdam, L. Toll, and G. Loew. 1988. Conformational determinants of high affinity δ receptor binding of opioid peptides. Mol. Pharmacol. 33:528–536.</ref> <ref>Kolp, B., F. Andreae, W. M. F. Fabian, and H. Sterk. 1996. Combined use of NMR, distance geometry and MD calculations for the conformational analysis of opioid peptides of the type [D(L)-Cys2, D(L)-Cys5]enkephalin. Int. J. Pept. Protein Res. 48:443–451.</ref> <ref>Lomize, A. L., I. D. Pogozheva, and H. I. Mosberg. 1996. Development of a model for the δ-opioid receptor pharmacophore. 3. Comparison of the cyclic tetrapeptide Tyr-c[D-Cys-Phe-D-Pen]OH with other conformationally constrained δ-receptor selective ligands. Biopolymers. 38:221–234.</ref> <ref>Mosberg, H. I. 1999. Complementarity of δ opioid ligand pharmacophore and receptor models. Biopolymers. 51:426–439.</ref> <ref>Shenderovitch, M. D., G. V. Nikiforovich, and A. A. Golbraikh. 1991. Conformational features responsible for the binding of cyclic analogues of enkephalin to opioid receptors. Int. J. Pept. Protein Res. 37:241–251.</ref> <ref>Tourwé, D., K. Verschueren, A. Frycia, P. Davis, F. Porreca, V. J. Hruby, G. Toth, H. Jaspers, P. Verheyden, and G. Van Binst. 1995. Conformational restriction of Tyr and Phe side chains in opioid peptides: information about preferred and bioactive side-chain topology. Biopolymers. 38:1–12.</ref> <ref>Wang, Y., and K. Kuczera. 1996. Molecular dynamics simulations of cyclic and linear DPDPE: influence of the disulfide bond on peptide flexibility. J. Phys. Chem. 100:2555–2563.</ref> <ref>Yamazaki, T., S. Ro, M. Goodman, N. N. Chung, and P. W. Schiller. 1993. A topochemical approach to explain morphiceptin bioactivity. J. Med. Chem. 36:708–719.</ref>. | ||
== Physiological functions == | == Physiological functions == | ||
Revision as of 20:31, 29 November 2021
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Borja Fernández García, Marina González Castilla, Michal Harel