We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Transmembrane protease serine 2
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
== General function == | == General function == | ||
| - | In terms of normal function, TMPRSS2 has | + | |
| - | been associated with physiological and pathological processes such as digestion, tissue remodelling, blood coagulation, fertility, inflammatory | + | In terms of normal function, TMPRSS2 has been associated with physiological and pathological processes such as digestion, tissue remodelling, blood coagulation, fertility, inflammatory responses, tumour cell invasion, apoptosis and pain. <ref>DOI 10.1097/j.pain.0000000000000130</ref> In the lung, it has been suggested that it regulates epithelial sodium currents through proteolytic cleavage of the epithelial sodium channel. However, knock out mice showed no obvious phenotypic abnormality such as death, infertility or visible sickness, and the exact physiological function of TMPRSS2 in vivo remains unknown. It is speculated that TMPRSS2 may contribute to a specialised but non vital function. <ref>DOI 10.1128/MCB.26.3.965-975.2006 </ref> |
| - | responses, tumour cell invasion, apoptosis and | + | |
| - | pain. <ref>DOI 10.1097/j.pain.0000000000000130</ref> In the lung, it has been suggested that it regulates epithelial sodium currents through proteolytic cleavage of the epithelial sodium channel. However, knock out mice showed no obvious phenotypic abnormality such as death, infertility or visible sickness, and the exact physiological function of TMPRSS2 in vivo remains unknown. It is speculated that TMPRSS2 may contribute to a specialised but non vital function. <ref>DOI 10.1128/MCB.26.3.965-975.2006 </ref> | + | |
=== Prostate cancer === | === Prostate cancer === | ||
| Line 31: | Line 29: | ||
====SARS-CoV-2==== | ====SARS-CoV-2==== | ||
| + | |||
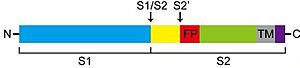
SARS-CoV-2 entry is achieved by a receptor-mediated endocytosis pathway in which the spike (S) glycoprotein, located on the outer envelope of the virus, interacts with the host angiotensin-converting enzyme 2 (ACE2), a receptor located in the surface of host cells, which allows the virus to infect cells. Prior to this interaction, S protein is needed to be cleaved by different protease enzymes (furins, cathepsins, serine proteases) <ref>DOI 10.1128/AAC.00754-20</ref>. SARS-CoV-2 S protein presents two functional domains S1, the receptor binding domain, and S2, that contains functional elements involved in membrane fusion. There are multiple sites in which this protein can be cleaved; one of these is at the S1/S2 boundary and another within S2. The S1/S2 cleavage site contains multiple arginine residues, which allows the action of serine proteases <ref>DOI 10.1136/jclinpath-2020-206987</ref>. ''Coronaviridae'' family tend to prefer "TMPRSS2" for the cleavage of S protein over other proteases, such as the endosomal cathepsins. As this protease is expressed in SARS-CoV-2 target cells throughout the human respiratory tract, it is also required for the spread of this virus. However, it has been demonstrated to be dispensable for the development or homeostasis of mice models, so it can be considered a potential target to fight the infection of these viruses. | SARS-CoV-2 entry is achieved by a receptor-mediated endocytosis pathway in which the spike (S) glycoprotein, located on the outer envelope of the virus, interacts with the host angiotensin-converting enzyme 2 (ACE2), a receptor located in the surface of host cells, which allows the virus to infect cells. Prior to this interaction, S protein is needed to be cleaved by different protease enzymes (furins, cathepsins, serine proteases) <ref>DOI 10.1128/AAC.00754-20</ref>. SARS-CoV-2 S protein presents two functional domains S1, the receptor binding domain, and S2, that contains functional elements involved in membrane fusion. There are multiple sites in which this protein can be cleaved; one of these is at the S1/S2 boundary and another within S2. The S1/S2 cleavage site contains multiple arginine residues, which allows the action of serine proteases <ref>DOI 10.1136/jclinpath-2020-206987</ref>. ''Coronaviridae'' family tend to prefer "TMPRSS2" for the cleavage of S protein over other proteases, such as the endosomal cathepsins. As this protease is expressed in SARS-CoV-2 target cells throughout the human respiratory tract, it is also required for the spread of this virus. However, it has been demonstrated to be dispensable for the development or homeostasis of mice models, so it can be considered a potential target to fight the infection of these viruses. | ||
| Line 56: | Line 55: | ||
== Expression == | == Expression == | ||
| + | |||
TMPRSS2 is predominantly expressed in prostate, with relatively lower level of expression in type II pneumocytes in lungs, colon, small intestine, stomach, salivary glands, liver, kidneys and páncreas. <ref>DOI </ref> | TMPRSS2 is predominantly expressed in prostate, with relatively lower level of expression in type II pneumocytes in lungs, colon, small intestine, stomach, salivary glands, liver, kidneys and páncreas. <ref>DOI </ref> | ||
| Line 65: | Line 65: | ||
===Regulation=== | ===Regulation=== | ||
| + | |||
The human TMPRSS2 gene promoter has a 15-bp androgen response element. The upregulation of TMPRSS2 mRNA by androgens appears to be mediated by the androgen receptor | The human TMPRSS2 gene promoter has a 15-bp androgen response element. The upregulation of TMPRSS2 mRNA by androgens appears to be mediated by the androgen receptor | ||
| Line 70: | Line 71: | ||
=== Inhibitors of TMPRSS2 === | === Inhibitors of TMPRSS2 === | ||
| + | |||
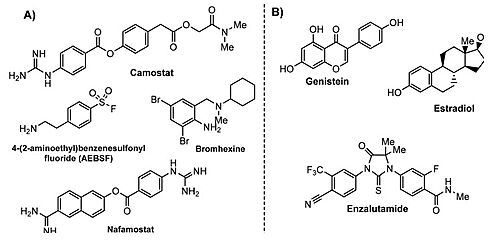
==== Nafamostat mesylate ==== | ==== Nafamostat mesylate ==== | ||
| Line 81: | Line 83: | ||
==== Camostat mesylate ==== | ==== Camostat mesylate ==== | ||
| + | |||
Camostat is a serine protease inhibitor used in the treatment of cancer, pancreatitis and liver fibrosis. A recent study demonstrated that the inhibition of TMPRSS2 with camostat led to a 10-fold reduction in SARS-CoV titers in Calu-3 cells. | Camostat is a serine protease inhibitor used in the treatment of cancer, pancreatitis and liver fibrosis. A recent study demonstrated that the inhibition of TMPRSS2 with camostat led to a 10-fold reduction in SARS-CoV titers in Calu-3 cells. | ||
==== Nafamostat vs. Camostat mesylate ==== | ==== Nafamostat vs. Camostat mesylate ==== | ||
| + | |||
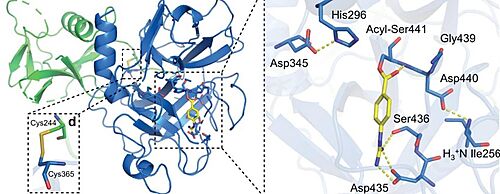
Nafamostat and Camostat are competitive inhibitors of the binding active site. They are both reactive esters that form the same slowly-reversible phenylguanidino covalent complex with the catalytic serine (Ser441) residue of trypsin-like serine proteases.<ref>DOI 10.1101/2021.06.23.449282</ref> However, Nafamostat demonstrated enhanced potency over camostat with IC50 values of (1.7±0.2) and (17±4) nM, respectively. Nafamostat has demonstrated being able to block MERS-CoV infection in vitro via inhabiting the activity of TMPRSS2, and reduce viral entry by 100-fold at a concentration of as low as 1 nM, which is more effective than camostat [74]. | Nafamostat and Camostat are competitive inhibitors of the binding active site. They are both reactive esters that form the same slowly-reversible phenylguanidino covalent complex with the catalytic serine (Ser441) residue of trypsin-like serine proteases.<ref>DOI 10.1101/2021.06.23.449282</ref> However, Nafamostat demonstrated enhanced potency over camostat with IC50 values of (1.7±0.2) and (17±4) nM, respectively. Nafamostat has demonstrated being able to block MERS-CoV infection in vitro via inhabiting the activity of TMPRSS2, and reduce viral entry by 100-fold at a concentration of as low as 1 nM, which is more effective than camostat [74]. | ||
Revision as of 10:43, 1 December 2021
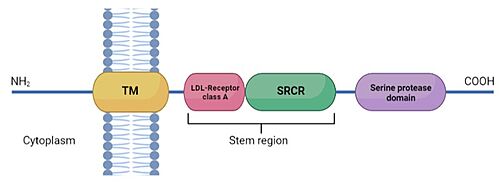
TMPRSS2 is a membrane protein belonging to the type II transmembrane serine protease (TTSP) family. It is functionally classified as a trypsin-like protease (TLP). [1] Serine proteases are known to be involved in many physiological and pathological processes.
| |||||||||||
References
- ↑ Sgrignani J, Cavalli A. Computational Identification of a Putative Allosteric Binding Pocket in TMPRSS2. Front Mol Biosci. 2021 Apr 30;8:666626. doi: 10.3389/fmolb.2021.666626., eCollection 2021. PMID:33996911 doi:http://dx.doi.org/10.3389/fmolb.2021.666626
- ↑ Evnin LB, Vasquez JR, Craik CS. Substrate specificity of trypsin investigated by using a genetic selection. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6659-63. doi: 10.1073/pnas.87.17.6659. PMID:2204062 doi:http://dx.doi.org/10.1073/pnas.87.17.6659
- ↑ Singh N, Decroly E, Khatib AM, Villoutreix BO. Structure-based drug repositioning over the human TMPRSS2 protease domain: search for chemical probes able to repress SARS-CoV-2 Spike protein cleavages. Eur J Pharm Sci. 2020 Oct 1;153:105495. doi: 10.1016/j.ejps.2020.105495. Epub, 2020 Jul 28. PMID:32730844 doi:http://dx.doi.org/10.1016/j.ejps.2020.105495
- ↑ Lam DK, Dang D, Flynn AN, Hardt M, Schmidt BL. TMPRSS2, a novel membrane-anchored mediator in cancer pain. Pain. 2015 May;156(5):923-930. doi: 10.1097/j.pain.0000000000000130. PMID:25734995 doi:http://dx.doi.org/10.1097/j.pain.0000000000000130
- ↑ Kim TS, Heinlein C, Hackman RC, Nelson PS. Phenotypic analysis of mice lacking the Tmprss2-encoded protease. Mol Cell Biol. 2006 Feb;26(3):965-75. doi: 10.1128/MCB.26.3.965-975.2006. PMID:16428450 doi:http://dx.doi.org/10.1128/MCB.26.3.965-975.2006
- ↑ St John J, Powell K, Conley-Lacomb MK, Chinni SR. TMPRSS2-ERG Fusion Gene Expression in Prostate Tumor Cells and Its Clinical and Biological Significance in Prostate Cancer Progression. J Cancer Sci Ther. 2012 Apr 26;4(4):94-101. doi: 10.4172/1948-5956.1000119. PMID:23264855 doi:http://dx.doi.org/10.4172/1948-5956.1000119
- ↑ Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005 Oct 28;310(5748):644-8. doi: 10.1126/science.1117679. PMID:16254181 doi:http://dx.doi.org/10.1126/science.1117679
- ↑ Carrere S, Verger A, Flourens A, Stehelin D, Duterque-Coquillaud M. Erg proteins, transcription factors of the Ets family, form homo, heterodimers and ternary complexes via two distinct domains. Oncogene. 1998 Jun 25;16(25):3261-8. doi: 10.1038/sj.onc.1201868. PMID:9681824 doi:http://dx.doi.org/10.1038/sj.onc.1201868
- ↑ Yu J, Yu J, Mani RS, Cao Q, Brenner CJ, Cao X, Wang X, Wu L, Li J, Hu M, Gong Y, Cheng H, Laxman B, Vellaichamy A, Shankar S, Li Y, Dhanasekaran SM, Morey R, Barrette T, Lonigro RJ, Tomlins SA, Varambally S, Qin ZS, Chinnaiyan AM. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010 May 18;17(5):443-54. doi: 10.1016/j.ccr.2010.03.018. PMID:20478527 doi:http://dx.doi.org/10.1016/j.ccr.2010.03.018
- ↑ Farooqi AA, Hou MF, Chen CC, Wang CL, Chang HW. Androgen receptor and gene network: Micromechanics reassemble the signaling machinery of TMPRSS2-ERG positive prostate cancer cells. Cancer Cell Int. 2014 Apr 17;14:34. doi: 10.1186/1475-2867-14-34. eCollection, 2014. PMID:24739220 doi:http://dx.doi.org/10.1186/1475-2867-14-34
- ↑ Thunders M, Delahunt B. Gene of the month: TMPRSS2 (transmembrane serine protease 2). J Clin Pathol. 2020 Dec;73(12):773-776. doi: 10.1136/jclinpath-2020-206987. Epub , 2020 Sep 1. PMID:32873700 doi:http://dx.doi.org/10.1136/jclinpath-2020-206987
- ↑ Hoffmann M, Schroeder S, Kleine-Weber H, Muller MA, Drosten C, Pohlmann S. Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19. Antimicrob Agents Chemother. 2020 May 21;64(6). pii: AAC.00754-20. doi:, 10.1128/AAC.00754-20. Print 2020 May 21. PMID:32312781 doi:http://dx.doi.org/10.1128/AAC.00754-20
- ↑ Thunders M, Delahunt B. Gene of the month: TMPRSS2 (transmembrane serine protease 2). J Clin Pathol. 2020 Dec;73(12):773-776. doi: 10.1136/jclinpath-2020-206987. Epub , 2020 Sep 1. PMID:32873700 doi:http://dx.doi.org/10.1136/jclinpath-2020-206987
- ↑ Thunders M, Delahunt B. Gene of the month: TMPRSS2 (transmembrane serine protease 2). J Clin Pathol. 2020 Dec;73(12):773-776. doi: 10.1136/jclinpath-2020-206987. Epub , 2020 Sep 1. PMID:32873700 doi:http://dx.doi.org/10.1136/jclinpath-2020-206987
- ↑ . PMID:216315890657
- ↑ Afar DE, Vivanco I, Hubert RS, Kuo J, Chen E, Saffran DC, Raitano AB, Jakobovits A. Catalytic cleavage of the androgen-regulated TMPRSS2 protease results in its secretion by prostate and prostate cancer epithelia. Cancer Res. 2001 Feb 15;61(4):1686-92. PMID:11245484
- ↑ Bertram S, Glowacka I, Blazejewska P, Soilleux E, Allen P, Danisch S, Steffen I, Choi SY, Park Y, Schneider H, Schughart K, Pohlmann S. TMPRSS2 and TMPRSS4 facilitate trypsin-independent spread of influenza virus in Caco-2 cells. J Virol. 2010 Oct;84(19):10016-25. doi: 10.1128/JVI.00239-10. Epub 2010 Jul 14. PMID:20631123 doi:http://dx.doi.org/10.1128/JVI.00239-10
- ↑ Shen LW, Mao HJ, Wu YL, Tanaka Y, Zhang W. TMPRSS2: A potential target for treatment of influenza virus and coronavirus infections. Biochimie. 2017 Nov;142:1-10. doi: 10.1016/j.biochi.2017.07.016. Epub 2017 Aug 1. PMID:28778717 doi:http://dx.doi.org/10.1016/j.biochi.2017.07.016
- ↑ Amraei R, Rahimi N. COVID-19, Renin-Angiotensin System and Endothelial Dysfunction. Cells. 2020 Jul 9;9(7). pii: cells9071652. doi: 10.3390/cells9071652. PMID:32660065 doi:http://dx.doi.org/10.3390/cells9071652
- ↑ Shrimp JH, Kales SC, Sanderson PE, Simeonov A, Shen M, Hall MD. An Enzymatic TMPRSS2 Assay for Assessment of Clinical Candidates and Discovery of Inhibitors as Potential Treatment of COVID-19. bioRxiv. 2020 Aug 6. doi: 10.1101/2020.06.23.167544. PMID:32596694 doi:http://dx.doi.org/10.1101/2020.06.23.167544
- ↑ Hitomi Y, Ikari N, Fujii S. Inhibitory effect of a new synthetic protease inhibitor (FUT-175) on the coagulation system. Haemostasis. 1985;15(3):164-8. doi: 10.1159/000215139. PMID:3161808 doi:http://dx.doi.org/10.1159/000215139
- ↑ doi: https://dx.doi.org/10.1101/2021.06.23.449282
- ↑ Maggio R, Corsini GU. Repurposing the mucolytic cough suppressant and TMPRSS2 protease inhibitor bromhexine for the prevention and management of SARS-CoV-2 infection. Pharmacol Res. 2020 Jul;157:104837. doi: 10.1016/j.phrs.2020.104837. Epub 2020, Apr 22. PMID:32334052 doi:http://dx.doi.org/10.1016/j.phrs.2020.104837
- ↑ doi: https://dx.doi.org/10.20944/preprints202003.0360.v2
Proteopedia Page Contributors and Editors (what is this?)
Paula R. Mallavibarrena, Ines Muniesa-Martinez, Michal Harel, Laura Aleixos Juan, Jaime Prilusky