User:Maria Carolina Boer Copstein/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 18: | Line 18: | ||
== Structural highlights == | == Structural highlights == | ||
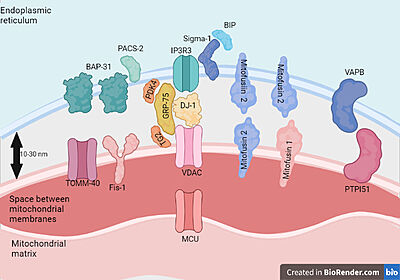
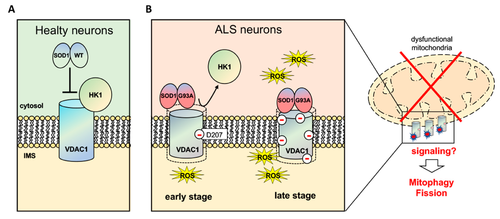
| - | Human VDAC1 (hVDAC1) adopts a β-barrel architecture composed of 19 <scene name='89/896608/Vdac1_helix/3'>β-strands</scene> with strands β1 and β19 being in parallel connformation and an <scene name='89/896608/Vdac1_helix/2'>α-helix</scene> located horizontally midway within the pore.<scene name='89/896608/Vdac1/3'>Ball and sticks structure</scene> N-terminal region of VDAC1, consisting of 25 amino acids,lies inside the channel pore and possesses different degrees of α-helical content in each of the three proposed structures.Various studies using purified rat liver , brain mitochondria or recombinant human VDAC1 have reported that both soluble purified and membrane-embedded VDAC1 can assemble into dimers, trimers, tetramers and higher oligomeric states. VDAC1 oligomerization was also demonstrated in VDAC1-reconstituted liposomes. | + | Human VDAC1 (hVDAC1) adopts a β-barrel architecture composed of 19 <scene name='89/896608/Vdac1_helix/3'>β-strands</scene> with strands β1 and β19 being in parallel connformation and an <scene name='89/896608/Vdac1_helix/2'>α-helix</scene> located horizontally midway within the pore.<scene name='89/896608/Vdac1/3'>Ball and sticks structure</scene> N-terminal region of VDAC1, consisting of 25 amino acids,lies inside the channel pore and possesses different degrees of α-helical content in each of the three proposed structures.Various studies using purified rat liver , brain mitochondria or recombinant human VDAC1 have reported that both soluble purified and membrane-embedded VDAC1 can assemble into <scene name='89/896608/Olig/1'>dimers</scene>, trimers, tetramers and higher oligomeric states. VDAC1 oligomerization was also demonstrated in VDAC1-reconstituted liposomes. |
Revision as of 04:04, 13 December 2021
| |||||||||||
References
Magri Andrea ,Messina Angela, “Interactions of VDAC with Proteins Involved in Neurodegenerative Aggregation: An Opportunity for Advancement on Therapeutic Molecules”, Current Medicinal Chemistry 2017; 24(40) . https://doi.org/10.2174/0929867324666170601073920