Malarial Dihydrofolate Reductase as Drug Target
From Proteopedia
| Line 10: | Line 10: | ||
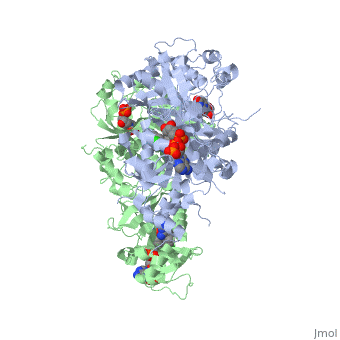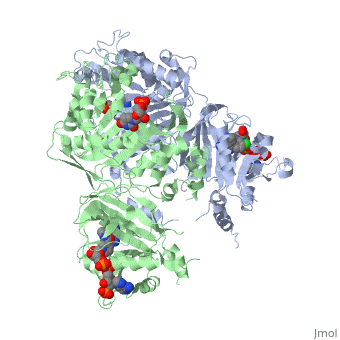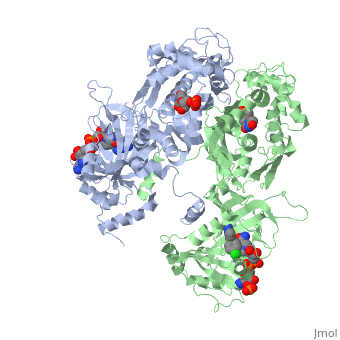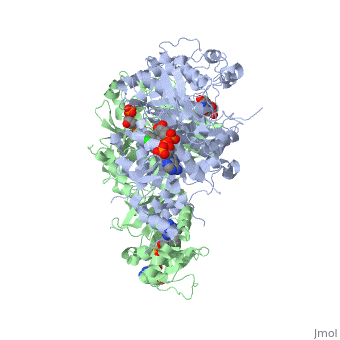
| - | [[Image:Fig1.jpeg|400px]]<ref name= | + | [[Image:Fig1.jpeg|400px]]<ref name="yu" >PMID:23035243</ref> |
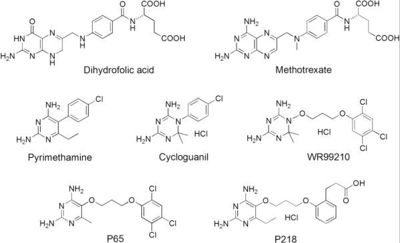
| - | A first step in this direction was to experiment with the drug <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Wr99210/1'>WR99210</scene>, a 4,6-diamino-1,2-dihydro-1,3,5-triazine that, with its (2,4,5-trichlorophenoxy)propoxy side chain, addressed the steric clash that <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Pyrimethamine_with_wt_pfdhfr/2'>pyrimethamine</scene> was subjected to with a Ser108Asn mutation. However further research with this drug was stopped because of its gastrointestinal toxicity in humans and low bioavailbility.<ref name= | + | A first step in this direction was to experiment with the drug <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Wr99210/1'>WR99210</scene>, a 4,6-diamino-1,2-dihydro-1,3,5-triazine that, with its (2,4,5-trichlorophenoxy)propoxy side chain, addressed the steric clash that <scene name='Malarial_Dihydrofolate_Reductase_as_Drug_Target/Pyrimethamine_with_wt_pfdhfr/2'>pyrimethamine</scene> was subjected to with a Ser108Asn mutation. However further research with this drug was stopped because of its gastrointestinal toxicity in humans and low bioavailbility.<ref name="yu"/> Wr99210, like cycloguanil, was significantly more basic than pyrimethamine, a pyrimidine and slightly acidic substance to match that of the gastrointestinal track, and thus was not readily absorbed in the intestines. When present in the gastrointestinal track, the triazines were fully protonated while the 2,4-diaminopyrimidines were present as a mixture of protonated and unprotonated. To test if protonation affected the bioavailability, a less basic compound with a 2,4-diaminopyrimidine scaffold was synthesized called P65. When it was compared via in vitro and in vivo experiments to Wr99210, P65 was shown to have significantly higher absorption in vivo. Thus, P65 was chosen as the basis for more research and the later discussed drug, P218. It was reasoned that protonation most likely interferes with absorption via passive diffusion and though Wr99210 was effective at getting deep into the mutant PfDHFR active site, its protonated state in the acidic gastrointestinal track would have made it unsuccessful as an oral drug, especially in comparison to its diaminopyrimidine analog, P65. |
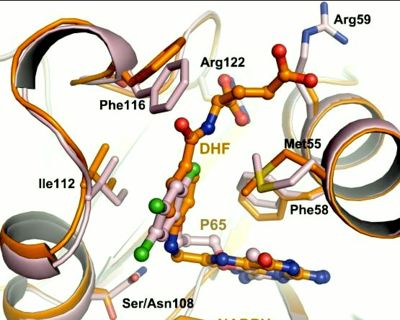
| - | [[Image:Fig22.jpeg|400px]] <ref name= | + | [[Image:Fig22.jpeg|400px]] <ref name="yu" /> |
| Line 28: | Line 28: | ||
| - | [[Image:Fig3.jpeg|400px]] <ref name= | + | [[Image:Fig3.jpeg|400px]] <ref name="yu"/> |
| - | In addition to the hydrogen bonding interactions by the P218 carboxyl group with Arg122, there are also strong hydrogen bonding interactions with another higher conserved residue, Phe 58. This interaction is another aid in helping P218 overcome resistance due to amino acid mutations in the active site. Phe58 is highly unlikely to be mutated because it would likely have adverse effects on the DHF substrate binding and negatively effect the functioning of the malaria protist. <ref name= | + | In addition to the hydrogen bonding interactions by the P218 carboxyl group with Arg122, there are also strong hydrogen bonding interactions with another higher conserved residue, Phe 58. This interaction is another aid in helping P218 overcome resistance due to amino acid mutations in the active site. Phe58 is highly unlikely to be mutated because it would likely have adverse effects on the DHF substrate binding and negatively effect the functioning of the malaria protist. <ref name="yu"/> During in vitro studies, P218 was shown to be very active against the quadruple mutant, pyrimethamine resistant PfDHFR. <ref name="yu"/> In vivo, it was also shown to be very active against quadruple mutant PfDHFR in SCID mice. <ref name="yu"/> This high level of inhibitor activity is also coupled with its success of being species selective and not causing any host toxicity at reasonable levels. However, there were experiments performed and it was found that threshold of adverse effects was about 100 mg/kg/d. <ref name="yu"/> |
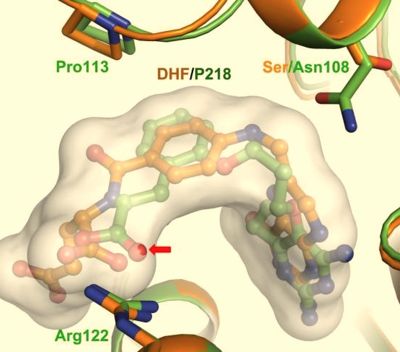
| - | [[Image:Fig4.jpeg|400px]] <ref name= | + | [[Image:Fig4.jpeg|400px]] <ref name="yu"/> |
| - | Due to P218 fitting well within the DHF substrate envelope, resistance to P218 would not be favorable to the organism because it could lead to adverse effects of DHF substrate binding and parasite fitness. <ref name= | + | Due to P218 fitting well within the DHF substrate envelope, resistance to P218 would not be favorable to the organism because it could lead to adverse effects of DHF substrate binding and parasite fitness. <ref name="yu"/> Additionally, the Ser/Asn108 mutation does not effect the binding of P218 like it would for pyrimethamine and its rigid chlorophenyl group, as mentioned previously. It's also shown that an oxygen of the carboxyl group of P218 slightly penetrates the DHF substrate envelope to allow for increased hydrogen bonding interaction with the highly conserved Arg122 and Phe58 residues over that of the DHF substrate. |
| Line 43: | Line 43: | ||
Conclusions | Conclusions | ||
---- | ---- | ||
| - | P218 has been determined to be an effective inhibitor of both wild-type and mutant, drug resistant PfDHFR, minimize host toxicity, and have reasonable bioavailability (46%).<ref name= | + | P218 has been determined to be an effective inhibitor of both wild-type and mutant, drug resistant PfDHFR, minimize host toxicity, and have reasonable bioavailability (46%).<ref name="yu"/> The key structural characteristics of the compound that allow for this are its pyrimidine side-chain flexibility and carboxylate group that forms hydrogen bonds selectively with the conserved arginine in PfDHFR. This ability to be of this inhibitor to overcome mutant forms of the enzyme and still be highly effective is really important to the future of antimalarial drugs. The species specific design is also greatly important to the drug in order to avoid host toxicity. P218 is thus a good candidate for further research as a malarial drug because of its shown promise to help to solve the current problem of drug resistant malaria effectively. |
</StructureSection> | </StructureSection> | ||
Revision as of 14:05, 7 January 2022
Introduction
Dihydrofolate reductase (DHFR) plays an essential role in the formation of DNA by managing folate, an organic molecule that shuttles carbons to enzymes that need them for their reactions. Relevant to this context of DHFR involvement, folate gives its carbons to thymidylate synthase, which then uses these carbons to make thymine bases. After folate has removed its carbon atoms, it is the job of DHFR to recycle it. It does this by transferring hydrogen atoms from NADPH to folate to restore it to its useful, reduced form.[1] DHFR is used in all organisms however, each organism makes a lsightly different version. The malaria version of the enzyme has shown to be reliable and the best target for antimalarial drugs. The current antimalarial drugs that target the malarial DHFR include pyrimethamine and cycloguanil. Pyrimethamine is currently used in combination sulfadoxine, a sulfadrug, to treat and prevent malaria but can result in serious side effects including liver damage.[2][3] Recently the effectiveness of these drugs has decreased because of mutations in the enzyme that have led to drug resistance. Since these mutations are becoming much more prevalent in malaria cases, new research in drug development must now incorporate both the wild-type as well as the quadruple mutant DHFR from the Plasmodium falciparum malarial strain, the most common and lethal of the malaria species.[4]
| |||||||||||
References
- ↑ Goodsell, David. "Dihydrofolate Reductase." RCSB PDB-101. RCSB PDB, Oct. 2002. Web. <http://www.rcsb.org/pdb/101/motm.do?momID=34>.
- ↑ "Pyrimethamine & Sulfadoxine." United States National Library of Medicine, n.d. Web. <http://livertox.nih.gov/PyrimethamineSulfadoxine.htm>.
- ↑ "Pyrimethamine and Sulfadoxine (Oral Route)." Mayo Clinic. Mayo Foundation for Medical Education and Research, 01 Nov. 2011. Web. <http://www.mayoclinic.com/health/drug-information/DR600357>.
- ↑ Somsak V, Uthaipibull C, Prommana P, Srichairatanakool S, Yuthavong Y, Kamchonwongpaisan S. Transgenic Plasmodium parasites stably expressing Plasmodium vivax dihydrofolate reductase-thymidylate synthase as in vitro and in vivo models for antifolate screening. Malar J. 2011 Oct 7;10:291. PMID: 21981896
- ↑ Huang F, Tang L, Yang H, Zhou S, Liu H, Li J, Guo S. Molecular epidemiology of drug resistance markers of Plasmodium falciparum in Yunnan Province, China. Malar J. 2012 Jul 28;11:243. PMID: 22839209
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. doi:, 10.1073/pnas.1204556109. Epub 2012 Oct 3. PMID:23035243 doi:http://dx.doi.org/10.1073/pnas.1204556109
- ↑ Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.
Proteopedia Page Contributors and Editors (what is this?)
Mary Smith, Alexander Berchansky, Karsten Theis, Michal Harel