Enkephalin
From Proteopedia
| Line 49: | Line 49: | ||
The second approach to increase enkephalin stability ''in-vivo'' is the '''blocking of the peptidases''' themselves, such as enkephalinase and aminopeptidase. One example is DENKIs <ref name="cullen"/>.Dual Enkephalinase Inhibitors (DENKIs) are a novel therapeutic approach for opioid use disorders. This class of compounds physiologically activates the endogenous opioid system by inhibiting the enzymes responsible for the breakdown of enkephalins, protecting endogenous enkephalins, increasing their half-lives and physiological actions <ref>Alvarez-Perez, B, Poras, H, Maldonado, R. THE INHIBITION OF ENKEPHALIN CATABOLISM BY DUAL ENKEPHALINASE INHIBITOR: A NOVEL POSSIBLE THERAPEUTIC APPROACH FOR OPIOID USE DISORDERS. Br J Pharmacol. 2021. Accepted Author Manuscript. https://doi.org/10.1111/bph.15656</ref>. | The second approach to increase enkephalin stability ''in-vivo'' is the '''blocking of the peptidases''' themselves, such as enkephalinase and aminopeptidase. One example is DENKIs <ref name="cullen"/>.Dual Enkephalinase Inhibitors (DENKIs) are a novel therapeutic approach for opioid use disorders. This class of compounds physiologically activates the endogenous opioid system by inhibiting the enzymes responsible for the breakdown of enkephalins, protecting endogenous enkephalins, increasing their half-lives and physiological actions <ref>Alvarez-Perez, B, Poras, H, Maldonado, R. THE INHIBITION OF ENKEPHALIN CATABOLISM BY DUAL ENKEPHALINASE INHIBITOR: A NOVEL POSSIBLE THERAPEUTIC APPROACH FOR OPIOID USE DISORDERS. Br J Pharmacol. 2021. Accepted Author Manuscript. https://doi.org/10.1111/bph.15656</ref>. | ||
| - | == [[Enkephalin 3D structures]] | + | == [[Enkephalin 3D structures]]== |
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
General functionEnkephalins were the first reported evidence of endogenous opioids in the brain, by John Hughes and Hans Kosterlitz in 1975 [1] [2]. They are pentapeptides that can be divided into two groups based on their carboxy-terminal amino acids: methionine-enkephalin and leucine-enkephalin. acts as a neurotransmitter through opioid receptors, more specifically through the classical opioid receptor δ [3]. The main functions of enkephalins include analgesia, but they are also involved in the control of respiratory, cardiovascular and gastrointestinal functions, and participate in neuroendocrine regulation [4] [5] [6]. Enkephalin is generated from the cleavage of the precursor pro-enkephalin, resulting in Met-enkephalin or Leu-enkephalin. The processing of one molecule of pro-enkephalin generates six copies of Met-enkephalin and one copy of Leu-enkephalin [3]. Enkephalin is mainly distributed throughout the central, peripheral and autonomic nervous system in mammals [3]. However, opioid receptors are broadly distributed in the body, such as the cardiac and gastrointestinal systems. StructureThe main conformations of enkephalin found in crystals have been classified in three categories, described as “extended,” “single bend,” and “double bend.”[7]. The picture below represents molecular models of Leu-enkephalin in the three main conformations found in solid state determinations when the peptide is dissolved in a DMSO/water cryomixture at 275 K. [8] [9]. 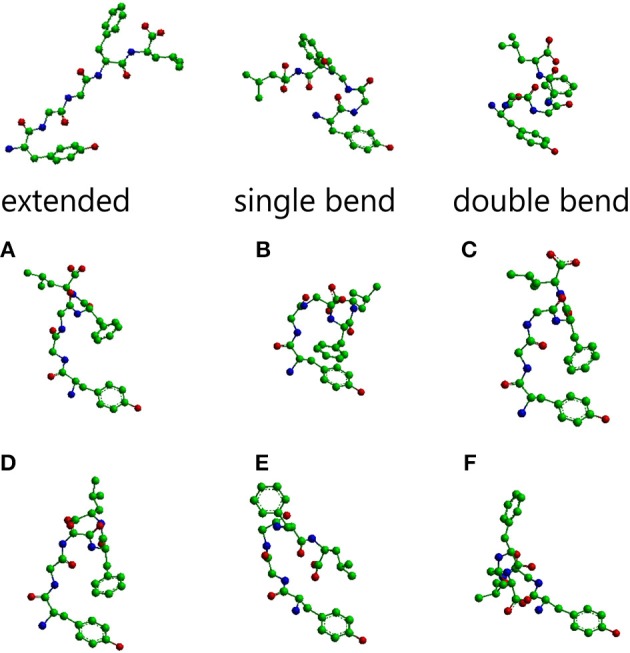 Molecular models of Leu-enkephalin in the three main conformations. Figure 1 from Sanfelice et al., Front. Mol. Biosci., https://doi.org/10.3389/fmolb.2014.00014 Met-enkephalin amino acid sequence is Tyr-Gly-Gly-Phe-Met, while leu-enkephalin amino acid sequence is Tyr-Gly-Gly-Phe-Leu. As we can see, of leu-enkephalin are on opposite sides of the backbone and point in different directions. A similar conformation was found for met-enkephalin in Bic/PG [4]. Variations in membrane composition seem to have an effect on the conformation adopted by enkephalins [4]. There is common agreement that the orientation of the tyrosine and phenylalanine rings with respect to each other dictates the receptor subtype selectivity [4]. It was originally believed that the μ-selective opiates adopted a folded conformation whereas the δ-opiates preferred an extended form [4] [10]. However, later other studies suggested a folded conformation with the Tyr and Phe aromatic rings in proximity for the δ-selective opiates, whereas the aromatic rings would point in different directions in the μ-type opiates [4] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] [21]. Physiological functionsAnalgesiaEnkephalins belong to one of the four major families of endogenous opioid ligands [22]. Opioid receptors couple to inhibitory G proteins and when they are activated Gα and Gβγ subunits dissociate and induce a signaling cascade that leads to a reduced neurotransmitter release [22]. All four opioid receptors inhibit N-, P/Q- and L-type voltage-gated calcium channels [22] [23] by the Gβγ subunit, which inhibits the entry of calcium to the pre-synaptic neuron, preventing the fusion of calcium-dependent synaptic vesicules with the membrane terminal and therefore blocking the neurotransmitter release. Transmission of pain signals is thus blocked. Enkephalins can be released from infiltrating immune cells at the site of injuries and from neurons in the central nervous system [22]. Stress response regulationSeveral studies showed the importance of enkephalins in anxiety and stress. A polymorphism in the gene encoding neutral endopeptidase involved in enkephalin metabolism, was identified in patients with anxiety disorders [24] [25]. Moreover, an enkephalin KO mice model had increased anxiety with the elevated plus maze (EPM), open field (OF) and light-dark box (LDB) tests, as well as an exaggerated startle response (SR), which is an unconscious defensive response to unexpected or threatened stimuli, and a reduced duration in the social interaction test (SI) [24] [26] [27] [28]. This suggests that a reduced enkephalin neurotransmission relates with the expression of anxiety. However, in other studies it appears that enkephalins enhanced the reactivity to chronic stress, as enkephalin KO mice were resistant to anxiety and depression-like behaviors after a chronic mild unpredictable stress [24] [29]. It seems that enkephalins have different effects on anxiety and stress and this might depend on the central nervous system region. In response to stress, Corticotropin-Releasing Factor (CRF) is released and stimulates the production of endogenous opioids such as endorphins and enkephalins. Enkephalin has been found to modulate the release of CRF from the paraventricular nucleus of the hypothalamus [3]. More knowledge on enkephalin pathways and their role is needed in order to fully understand stress regulation and a variety of stress- and anxiety- related disorders. Formation of social memoryEnkephalin has a depressant or inhibitory function of neuronal communication, and it is produced by VIP neurons [30], located in a small area of the hippocampus called CA2, which is known to be involved in the formation of social memory [31]. In mice, it is observed that VIP neurons show greater activity during the encounter with an unknown individual than against another relative and also when faced with a new object [32]. With the release of enkephalin by VIP interneurons in CA2, a special type of plasticity is induced, called ITDP (Input-timing-dependent plasticity), which is essential for the formation of social memory [33]. CA2 dysfunction has been linked to schizophrenia in humans and is believed to contribute to social memory deficits. We know from mouse models of schizophrenia that the CA2 zone is poorly regulated in this pathology, and that these mice cannot form social memory. This group is currently studying how enkephalin-mediated plasticity can be used to rescue the ability to form social memory in these animals. PathophysiologyDue to the wide distribution of the endogenous opioid system in the human body, its dysregulation has a high number of implications. One example of pain dysregulation is fibromyalgia, which is a widespread pain in the absence of identifiable peripheral pathology [34]. It appears that the endogenous opioid system is involved in some aspects of pain in fibromyalgia [35]. The concentration of endogenous opioids in the cerebrospinal fluid (CSF) of patients with fibromyalgia seems to be higher, which leads to a decreased availability and affinity of Mu-opioid receptors (MOR) [35]. This translates into an increase in pain neurotransmission. The enkephalin signaling pathway regulates various neural functions and can be altered by neurodegenerative disorders. In Alzheimer's disease (AD), elevated enkephalin levels may reflect compensatory processes or contribute to cognitive impairments. The therapeutic potential of reducing enkephalin production or signaling merits further exploration[36]. Decreased plasma met-enkephalin levels are vasogenic mediators in Systemic Sclerosis (SSc), and will associate with clinical manifestations of SSc-related vascular and fibrogenic injuries[37]. Proenkephalin (PENK) represents a novel biomarker for kidney function. PENK plasma concentration appears to accurately represent glomerular filtration rate in patients diagnosed with sepsis or cardiac diseases. Increased PENK concentration is found to be associated with Accurate Kidney Injury and cardiac diseases. Moreover, the predominant receptor of enkephalins, the δ-opioid receptor, is expressed with the highest density in the kidney, suggesting that enkephalins could also exert a direct effect on kidney function[38]. Clinical relevanceMet-Enkephalin (MENK) may contribute to immune responses against tumors and viral infections by activating multiple types of immune cells, enabling them to secrete various cytokines or directly kill target cells. The nuclear membrane of certain cancer cells expressed receptors to which MENK bound, resulting in marked growth inhibition of cancer cells in vitro[39]. Activation of δ opioid receptors acts against oxidative stress in the central nervous system and promotes neuron survival. Enkephalin is able to cross the blood-brain barrier; it has been hypothesized to be a promising strategy to promote neuron regeneration, especially in brain damage caused by stroke [40]. Multifunctional fluorinated enkephalin analog, LYS739 can be considered as a potential lead for ischemic stroke research and may provide advantages given the multimeric peptide-opiate structure[41]. The main and most known effect of enkephalins is analgesia, which makes the therapeutic use of enkephalins to treat pain one their main clinical relevant aspect. However, enkephalins have a relatively low stability in-vivo, due to their degradation by endogenous peptidases [3]. Two approaches have been proposed to this issue: the first one is the chemical modification of enkephalins while preserving their analgesic efficacy. One example of this is the design of D-Ala-methionine-enkephalin in 1976 by Pert et al. [42]. Kropotova et al. in 2020 have designed different modified enkephalins that are less accessible to endopeptidases [43]. Squalene-based nanoparticles have opened exciting perspectives for drug delivery due to their biodegradability and their non-toxicity as Leu-Enkephalin Nanomedicines for pain alleviation. LENK-SQ bioconjugates show exclusively peripheral activity (no BBB penetration) so no CNS addiction and take advantage of the inflammatory process to optimize drug concentrations at the site of injury [44]. The second approach to increase enkephalin stability in-vivo is the blocking of the peptidases themselves, such as enkephalinase and aminopeptidase. One example is DENKIs [3].Dual Enkephalinase Inhibitors (DENKIs) are a novel therapeutic approach for opioid use disorders. This class of compounds physiologically activates the endogenous opioid system by inhibiting the enzymes responsible for the breakdown of enkephalins, protecting endogenous enkephalins, increasing their half-lives and physiological actions [45]. Enkephalin 3D structuresReferences
| ||||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Borja Fernández García, Marina González Castilla, Michal Harel
