|
|
| Line 1: |
Line 1: |
| | | | |
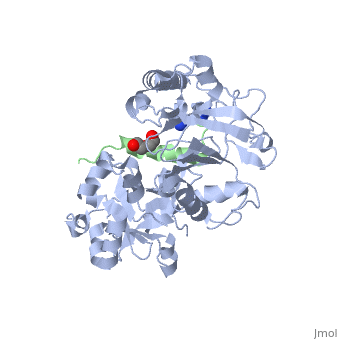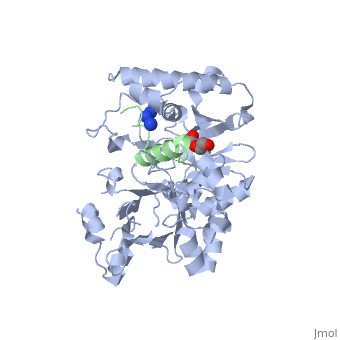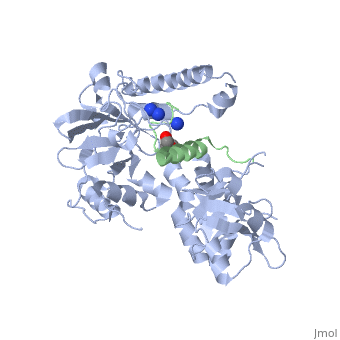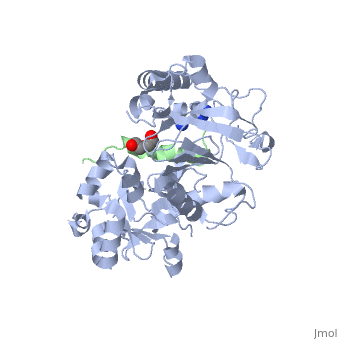
| | ==Crystal structure of Agrobacterium tumefaciens VirE2 in complex with its chaperone VirE1: a novel fold and implications for DNA binding== | | ==Crystal structure of Agrobacterium tumefaciens VirE2 in complex with its chaperone VirE1: a novel fold and implications for DNA binding== |
| - | <StructureSection load='3btp' size='340' side='right' caption='[[3btp]], [[Resolution|resolution]] 2.30Å' scene=''> | + | <StructureSection load='3btp' size='340' side='right'caption='[[3btp]], [[Resolution|resolution]] 2.30Å' scene=''> |
| | == Structural highlights == | | == Structural highlights == |
| - | <table><tr><td colspan='2'>[[3btp]] is a 2 chain structure with sequence from [http://en.wikipedia.org/wiki/Agrfc Agrfc]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=3BTP OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=3BTP FirstGlance]. <br> | + | <table><tr><td colspan='2'>[[3btp]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/Agrfc Agrfc]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=3BTP OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=3BTP FirstGlance]. <br> |
| - | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=NH4:AMMONIUM+ION'>NH4</scene>, <scene name='pdbligand=PEG:DI(HYDROXYETHYL)ETHER'>PEG</scene></td></tr> | + | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=NH4:AMMONIUM+ION'>NH4</scene>, <scene name='pdbligand=PEG:DI(HYDROXYETHYL)ETHER'>PEG</scene></td></tr> |
| - | <tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">virE2, Atu6190, AGR_pTi_28 ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=176299 AGRFC]), virE1, Atu6189, AGR_pTi_26 ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=176299 AGRFC])</td></tr> | + | <tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">virE2, Atu6190, AGR_pTi_28 ([https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=176299 AGRFC]), virE1, Atu6189, AGR_pTi_26 ([https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=176299 AGRFC])</td></tr> |
| - | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=3btp FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3btp OCA], [http://pdbe.org/3btp PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=3btp RCSB], [http://www.ebi.ac.uk/pdbsum/3btp PDBsum], [http://prosat.h-its.org/prosat/prosatexe?pdbcode=3btp ProSAT]</span></td></tr> | + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=3btp FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3btp OCA], [https://pdbe.org/3btp PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=3btp RCSB], [https://www.ebi.ac.uk/pdbsum/3btp PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=3btp ProSAT]</span></td></tr> |
| | </table> | | </table> |
| | == Function == | | == Function == |
| - | [[http://www.uniprot.org/uniprot/VIRE2_AGRT5 VIRE2_AGRT5]] Involved in DNA transformation; mediates the nuclear uptake of single-stranded DNA copies of the transferred DNA (T-DNA) element. Binds single-stranded but not double-stranded DNA regardless of nucleotide sequence composition.<ref>PMID:17784072</ref> <ref>PMID:8637884</ref> <ref>PMID:12124400</ref> [[http://www.uniprot.org/uniprot/VIRE1_AGRT5 VIRE1_AGRT5]] Involved in DNA transformation; controls virE2 polymerization and prevents virE2 binding to DNA. | + | [[https://www.uniprot.org/uniprot/VIRE2_AGRT5 VIRE2_AGRT5]] Involved in DNA transformation; mediates the nuclear uptake of single-stranded DNA copies of the transferred DNA (T-DNA) element. Binds single-stranded but not double-stranded DNA regardless of nucleotide sequence composition.<ref>PMID:17784072</ref> <ref>PMID:8637884</ref> <ref>PMID:12124400</ref> [[https://www.uniprot.org/uniprot/VIRE1_AGRT5 VIRE1_AGRT5]] Involved in DNA transformation; controls virE2 polymerization and prevents virE2 binding to DNA. |
| | == Evolutionary Conservation == | | == Evolutionary Conservation == |
| | [[Image:Consurf_key_small.gif|200px|right]] | | [[Image:Consurf_key_small.gif|200px|right]] |
| Line 31: |
Line 31: |
| | | | |
| | ==See Also== | | ==See Also== |
| - | *[[DNA-binding protein VirE2 from Agrobacterium tumefaciens complexed with chaperone VirE1|DNA-binding protein VirE2 from Agrobacterium tumefaciens complexed with chaperone VirE1]] | |
| - | *[[Interactive 3D Complements in Proteopedia|Interactive 3D Complements in Proteopedia]] | |
| | *[[VirE1-VirE2|VirE1-VirE2]] | | *[[VirE1-VirE2|VirE1-VirE2]] |
| | == References == | | == References == |
| Line 39: |
Line 37: |
| | </StructureSection> | | </StructureSection> |
| | [[Category: Agrfc]] | | [[Category: Agrfc]] |
| | + | [[Category: Large Structures]] |
| | [[Category: Albeck, S]] | | [[Category: Albeck, S]] |
| | [[Category: Dym, O]] | | [[Category: Dym, O]] |
| Structural highlights
3btp is a 2 chain structure with sequence from Agrfc. Full crystallographic information is available from OCA. For a guided tour on the structure components use FirstGlance.
| | Ligands: | , |
| Gene: | virE2, Atu6190, AGR_pTi_28 (AGRFC), virE1, Atu6189, AGR_pTi_26 (AGRFC) |
| Resources: | FirstGlance, OCA, PDBe, RCSB, PDBsum, ProSAT |
Function
[VIRE2_AGRT5] Involved in DNA transformation; mediates the nuclear uptake of single-stranded DNA copies of the transferred DNA (T-DNA) element. Binds single-stranded but not double-stranded DNA regardless of nucleotide sequence composition.[1] [2] [3] [VIRE1_AGRT5] Involved in DNA transformation; controls virE2 polymerization and prevents virE2 binding to DNA.
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
Publication Abstract from PubMed
Agrobacterium tumefaciens infects its plant hosts by a mechanism of horizontal gene transfer. This capability has led to its widespread use in artificial genetic transformation. In addition to DNA, the bacterium delivers an abundant ssDNA binding protein, VirE2, whose roles in the host include protection from cytoplasmic nucleases and adaptation for nuclear import. In Agrobacterium, VirE2 is bound to its acidic chaperone VirE1. When expressed in vitro in the absence of VirE1, VirE2 is prone to oligomerization and forms disordered filamentous aggregates. These filaments adopt an ordered solenoidal form in the presence of ssDNA, which was characterized previously by electron microscopy and three-dimensional image processing. VirE2 coexpressed in vitro with VirE1 forms a soluble heterodimer. VirE1 thus prevents VirE2 oligomerization and competes with its binding to ssDNA. We present here a crystal structure of VirE2 in complex with VirE1, showing that VirE2 is composed of two independent domains presenting a novel fold, joined by a flexible linker. Electrostatic interactions with VirE1 cement the two domains of VirE2 into a locked form. Comparison with the electron microscopy structure indicates that the VirE2 domains adopt different relative orientations. We suggest that the flexible linker between the domains enables VirE2 to accommodate its different binding partners.
Crystal structure of the Agrobacterium virulence complex VirE1-VirE2 reveals a flexible protein that can accommodate different partners.,Dym O, Albeck S, Unger T, Jacobovitch J, Branzburg A, Michael Y, Frenkiel-Krispin D, Wolf SG, Elbaum M Proc Natl Acad Sci U S A. 2008 Aug 12;105(32):11170-5. Epub 2008 Aug 4. PMID:18678909[4]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Citovsky V, DE Vos G, Zambryski P. Single-Stranded DNA Binding Protein Encoded by the virE Locus of Agrobacterium tumefaciens. Science. 1988 Apr 22;240(4851):501-4. PMID:17784072 doi:10.1126/science.240.4851.501
- ↑ Zupan JR, Citovsky V, Zambryski P. Agrobacterium VirE2 protein mediates nuclear uptake of single-stranded DNA in plant cells. Proc Natl Acad Sci U S A. 1996 Mar 19;93(6):2392-7. PMID:8637884
- ↑ Tzfira T, Vaidya M, Citovsky V. Increasing plant susceptibility to Agrobacterium infection by overexpression of the Arabidopsis nuclear protein VIP1. Proc Natl Acad Sci U S A. 2002 Aug 6;99(16):10435-40. Epub 2002 Jul 17. PMID:12124400 doi:10.1073/pnas.162304099
- ↑ Dym O, Albeck S, Unger T, Jacobovitch J, Branzburg A, Michael Y, Frenkiel-Krispin D, Wolf SG, Elbaum M. Crystal structure of the Agrobacterium virulence complex VirE1-VirE2 reveals a flexible protein that can accommodate different partners. Proc Natl Acad Sci U S A. 2008 Aug 12;105(32):11170-5. Epub 2008 Aug 4. PMID:18678909
|