Sandbox Reserved 1716
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
<scene name='90/904321/Closedconformation/2'>Spinning VKOR</scene> | <scene name='90/904321/Closedconformation/2'>Spinning VKOR</scene> | ||
[https://en.wikipedia.org/wiki/Vitamin_K_epoxide_reductase VKOR WIKI] | [https://en.wikipedia.org/wiki/Vitamin_K_epoxide_reductase VKOR WIKI] | ||
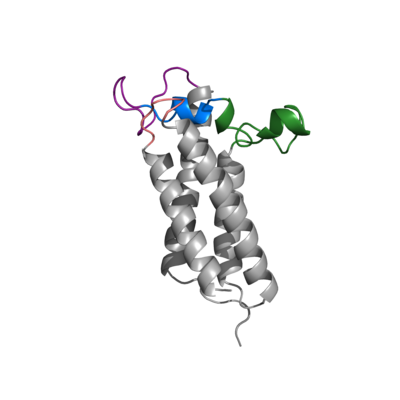
| - | + | The VKOR enzyme is made up of four transmembrane helices: T1, T2, T3, and T4.(Pictured in Grey) Each of these helices come together to form a hydrophobic pocket, that is topped by a cap domain. In the cap domain are important regions that are significant for Vitamin K binding, and the overall function of Vitamin K Epoxide Reductase. These important regions are the Anchor(Green), Cap Region (Blue), Beta Hairpin (Purple), and 3-4 Loop (Pink). | |
| + | |||
| + | <ref name="Ransey">PMID:28504306</ref> | ||
===Transmembrane Helices=== | ===Transmembrane Helices=== | ||
===Cap Domain=== | ===Cap Domain=== | ||
Revision as of 15:42, 24 March 2022
Vitamin K Epoxide Reductase
| |||||||||||
References
- ↑ Ransey E, Paredes E, Dey SK, Das SR, Heroux A, Macbeth MR. Crystal structure of the Entamoeba histolytica RNA lariat debranching enzyme EhDbr1 reveals a catalytic Zn(2+) /Mn(2+) heterobinucleation. FEBS Lett. 2017 Jul;591(13):2003-2010. doi: 10.1002/1873-3468.12677. Epub 2017, Jun 14. PMID:28504306 doi:http://dx.doi.org/10.1002/1873-3468.12677