We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1725
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
===Catalytic Cysteines=== | ===Catalytic Cysteines=== | ||
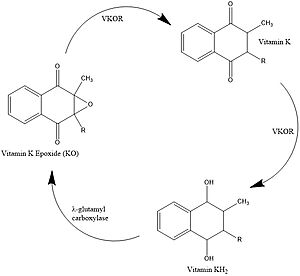
| - | VKOR uses four catalytic cysteines (43, 51, 132, and 135) to facilitate reduction and cause conformational changes via disulfide bridge formation. When an oxidized or partially oxidized Vitamin K enters the active site, VKOR has a stabilizing | + | VKOR uses four catalytic cysteines (43, 51, 132, and 135) to facilitate reduction and cause conformational changes via disulfide bridge formation. When an oxidized or partially oxidized Vitamin K enters the active site, VKOR has a stabilizing C51-C132 disulfide bond. C135 is not bound and is available to help Vitamin K bind. C43 attacks the C51-C132 bond, forming a new C43-C51 bond. The free amino acid at the 132 position is shown as a serine in this depiction because the researchers mutated it to stop the reaction at this intermediate. In the wild type VKOR, the 132 position would be a cysteine. C132 forms a bridge with C135 which allows release of the reduced or partially reduced Vitamin K. These rearrangements facilitate the use of cysteines as reducing agents for Vitamin K. Cysteine rearrangements also contribute to and are affected by overall conformational changes which affects their proximity to each other and the active site. |
| - | + | ||
| + | |||
| + | If we go back to State 2, when Vitamin K first binds, you can see that 135 is not tied up in a disulfide bond. It is available to help the Vitamin K bond. So, it makes sense that once 135 gets forced to bond, the now reduced Vitamin K is released. State 5 is interesting because the disulfide bonds are similar to the open state, but warfarin is actually bound. This represents the binding of warfarin to the fully oxidized VKOR at the end of its cycle. Going back to State 1, the researchers used a mutation at 43 to mimic VKOR’s partially oxidized state. Warfarin can also bind to this state and notice that the disulfide bonds are the same as State 2. Also it is worth pointing out how the disulfide bonds contribute to conformational changes and are affected by conformational changes, which affects their proximity to each other and the active site. | ||
Revision as of 20:34, 24 March 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
Vitamin K Epoxide Reductase
| |||||||||||
References
- ↑ Stafford DW. The vitamin K cycle. J Thromb Haemost. 2005 Aug;3(8):1873-8. doi: 10.1111/j.1538-7836.2005.01419.x. PMID:16102054 doi:http://dx.doi.org/10.1111/j.1538-7836.2005.01419.x
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
Student Contributors
Izabella Jordan, Emma Varness