Sandbox Reserved 1713
From Proteopedia
| Line 5: | Line 5: | ||
<StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> | ||
== Structure & Function == | == Structure & Function == | ||
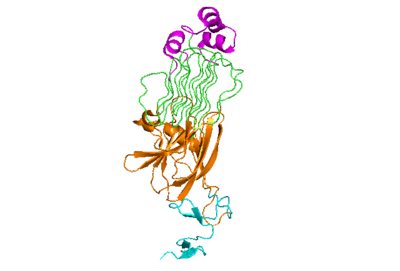
| - | The active state of the kinase is when two monomers have completed a conformational change and moved across the membrane to form a <scene name='90/904317/Dimer_full_colored/3'>dimer</scene>. | ||
===Domains=== | ===Domains=== | ||
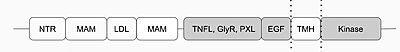
[[Image:2d_image.jpg|400 px|center|thumb|Fig.1 Anaplastic Lymphoma Kinase and its domains. The region from NTR to the MAM is the Heparin Binding Domain. The TNFL-PXL are the extracellular domains and the EGF is the domain that binds the extracellular region with the extracellular region of the transmembrane. The TMH is the transmembrane domain. The kinase domain is the intracellular portion of the ALK.]] | [[Image:2d_image.jpg|400 px|center|thumb|Fig.1 Anaplastic Lymphoma Kinase and its domains. The region from NTR to the MAM is the Heparin Binding Domain. The TNFL-PXL are the extracellular domains and the EGF is the domain that binds the extracellular region with the extracellular region of the transmembrane. The TMH is the transmembrane domain. The kinase domain is the intracellular portion of the ALK.]] | ||
| Line 17: | Line 16: | ||
===Conformational Change=== | ===Conformational Change=== | ||
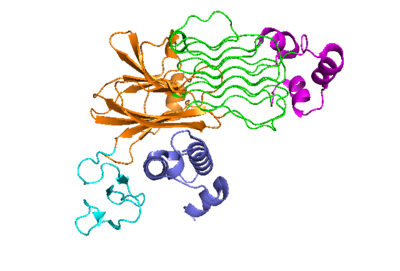
The anaplastic lymphoma kinase activating ligand (ALKAL) binds to the <scene name='90/904318/Alk-alkal_binding_surface/2'>binding surface</scene> on the ALK at the TNFL domain. This induces a conformational change which allows for the PXL and the GlyR domains to hinge forward.<ref>DOI: 10.1038/s41586-021-04140-8</ref> (Figure 2) ALK's TNFL has <scene name='90/904318/Binding_surface_with_residues/3'>residues</scene> E978, E974, E859, and Y966 that form salt bridges with R123, R133, R136, R140, and R117 on ALKAL that allow for activation, leading to <scene name='90/904317/Dimer_full_colored/3'>dimerization</scene> of two ALK-ALKAL monomers. | The anaplastic lymphoma kinase activating ligand (ALKAL) binds to the <scene name='90/904318/Alk-alkal_binding_surface/2'>binding surface</scene> on the ALK at the TNFL domain. This induces a conformational change which allows for the PXL and the GlyR domains to hinge forward.<ref>DOI: 10.1038/s41586-021-04140-8</ref> (Figure 2) ALK's TNFL has <scene name='90/904318/Binding_surface_with_residues/3'>residues</scene> E978, E974, E859, and Y966 that form salt bridges with R123, R133, R136, R140, and R117 on ALKAL that allow for activation, leading to <scene name='90/904317/Dimer_full_colored/3'>dimerization</scene> of two ALK-ALKAL monomers. | ||
| - | === | + | ===Membrane Guidance of ALKAL to ALK=== |
| - | The negatively charged phosphate groups on the cell membrane interact with | + | The negatively charged phosphate groups on the cell membrane interact with a highly conserved positively charged <scene name='90/904318/Alkalbindingsurfacewmembrane/1'>helix</scene> on ALKAL that faces the membrane. These <scene name='90/904318/Alkal1membraneinteraction/2'>residues that interact with the cell membrane</scene> guides ALKAL to ALK and correctly positions ALKAL for its binding surface to face ALK's binding surface, which allows for a more favorable interaction. <ref>DOI: 10.1038/s41586-021-04140-8</ref> |
== Disease == | == Disease == | ||
There are many mutations that could take place causing constitutive receptor activation, enhancement between the interaction of receptors or stabilization of active receptors are known to relate to oncogenic potentials. | There are many mutations that could take place causing constitutive receptor activation, enhancement between the interaction of receptors or stabilization of active receptors are known to relate to oncogenic potentials. | ||
Revision as of 04:02, 28 March 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
Anaplastic Lymphoma Kinase
Background
The anaplastic lymphoma kinase was first discovered in 1994 as a tyrosine kinase in a type of lymphoma cancer cell. ALK is a specific type of RTK which plays a huge role in transmembrane signaling and communication within the cell. ALK is commonly expressed in the development of the nervous system. Anaplastic Lymphoma Kinase receptor is a membrane-bound tyrosine kinase. The ALK transfers a phosphate group from ATP to a tyrosine residue on an enzyme which activates a signaling cascade, and ALK becomes activated when a ligand called ALKAL binds to the binding surface on an extracellular domain of ALK. ALK is an integral membrane protein. Abnormal forms of ALK are closely related to the formation of several cancers.
| |||||||||||
References
- ↑ Murray PB, Lax I, Reshetnyak A, Ligon GF, Lillquist JS, Natoli EJ Jr, Shi X, Folta-Stogniew E, Gunel M, Alvarado D, Schlessinger J. Heparin is an activating ligand of the orphan receptor tyrosine kinase ALK. Sci Signal. 2015 Jan 20;8(360):ra6. doi: 10.1126/scisignal.2005916. PMID:25605972 doi:http://dx.doi.org/10.1126/scisignal.2005916
- ↑ Reshetnyak AV, Rossi P, Myasnikov AG, Sowaileh M, Mohanty J, Nourse A, Miller DJ, Lax I, Schlessinger J, Kalodimos CG. Mechanism for the activation of the anaplastic lymphoma kinase receptor. Nature. 2021 Dec;600(7887):153-157. doi: 10.1038/s41586-021-04140-8. Epub 2021, Nov 24. PMID:34819673 doi:http://dx.doi.org/10.1038/s41586-021-04140-8
- ↑ Reshetnyak AV, Rossi P, Myasnikov AG, Sowaileh M, Mohanty J, Nourse A, Miller DJ, Lax I, Schlessinger J, Kalodimos CG. Mechanism for the activation of the anaplastic lymphoma kinase receptor. Nature. 2021 Dec;600(7887):153-157. doi: 10.1038/s41586-021-04140-8. Epub 2021, Nov 24. PMID:34819673 doi:http://dx.doi.org/10.1038/s41586-021-04140-8
- ↑ De Munck S, Provost M, Kurikawa M, Omori I, Mukohyama J, Felix J, Bloch Y, Abdel-Wahab O, Bazan JF, Yoshimi A, Savvides SN. Structural basis of cytokine-mediated activation of ALK family receptors. Nature. 2021 Oct 13. pii: 10.1038/s41586-021-03959-5. doi:, 10.1038/s41586-021-03959-5. PMID:34646012 doi:http://dx.doi.org/10.1038/s41586-021-03959-5
- ↑ Li T, Stayrook SE, Tsutsui Y, Zhang J, Wang Y, Li H, Proffitt A, Krimmer SG, Ahmed M, Belliveau O, Walker IX, Mudumbi KC, Suzuki Y, Lax I, Alvarado D, Lemmon MA, Schlessinger J, Klein DE. Structural basis for ligand reception by anaplastic lymphoma kinase. Nature. 2021 Dec;600(7887):148-152. doi: 10.1038/s41586-021-04141-7. Epub 2021, Nov 24. PMID:34819665 doi:http://dx.doi.org/10.1038/s41586-021-04141-7