Sandbox Reserved 1715
From Proteopedia
(Difference between revisions)
| Line 54: | Line 54: | ||
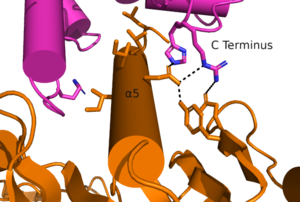
'''1.''' mGlu starts in an <scene name='90/904320/Inactive_mglu/2'>inactive homodimeric form</scene>. In this conformation, the receptor is considered open with an inter-lobe angle of 44 degrees.The structure has two free binding sites in the VFT, the CRDs are separated, and the TMD is not interacting with a G protein. [[Image: Protein Interaction with G Protein.png|300 px|right|thumb|Figure 2. The interaction between an active mGlu and a G-protein ]] | '''1.''' mGlu starts in an <scene name='90/904320/Inactive_mglu/2'>inactive homodimeric form</scene>. In this conformation, the receptor is considered open with an inter-lobe angle of 44 degrees.The structure has two free binding sites in the VFT, the CRDs are separated, and the TMD is not interacting with a G protein. [[Image: Protein Interaction with G Protein.png|300 px|right|thumb|Figure 2. The interaction between an active mGlu and a G-protein ]] | ||
| - | '''2.''' In the intermediate activation state (known as the open-closed conformation), one glutamate is bound in one binding pocket of VFT. This <scene name='90/904320/Mglu_binding/4'>glutamate bound state</scene> is still considered inactive as the receptor has not changed the conformations in the CRD and thus the TMD. With the same asymmetric transmembrane helices formation, a TM3-TM4 interface is still present and mGlu cannot interact with a G protein. | + | '''2.''' In the intermediate activation state (known as the open-closed conformation), one glutamate is bound in one binding pocket of VFT. This <scene name='90/904320/Mglu_binding/4'>glutamate bound state</scene> is still considered inactive as the receptor has not changed the conformations in the CRD and thus the TMD. With the same asymmetric transmembrane helices formation, a TM3-TM4 interface is still present and mGlu cannot interact with a G protein. |
'''3.''' A second glutamate binds to the other <scene name='90/904320/Active_site_interactions/3'>binding pocket</scene> of the VFT. Mediated by L639, F643, N735, W773, and F776, a positive allosteric modulator (PAM) also binds within the seven TMD helices of the alpha chain. This closed conformation with an inter-lobe domain of 25 degrees is considered the <scene name='90/904319/Pam/1'>active conformation</scene>. The binding of these ligands allows the CRD to compact and come together. This transformation causes the TMD to form another asymmetric conformation with a TM6-TM6 interface between the chains. | '''3.''' A second glutamate binds to the other <scene name='90/904320/Active_site_interactions/3'>binding pocket</scene> of the VFT. Mediated by L639, F643, N735, W773, and F776, a positive allosteric modulator (PAM) also binds within the seven TMD helices of the alpha chain. This closed conformation with an inter-lobe domain of 25 degrees is considered the <scene name='90/904319/Pam/1'>active conformation</scene>. The binding of these ligands allows the CRD to compact and come together. This transformation causes the TMD to form another asymmetric conformation with a TM6-TM6 interface between the chains. | ||
Revision as of 05:54, 28 March 2022
Metabotropic Glutamate Receptor
| |||||||||||
References
- ↑ 1.0 1.1 Ransey E, Paredes E, Dey SK, Das SR, Heroux A, Macbeth MR. Crystal structure of the Entamoeba histolytica RNA lariat debranching enzyme EhDbr1 reveals a catalytic Zn(2+) /Mn(2+) heterobinucleation. FEBS Lett. 2017 Jul;591(13):2003-2010. doi: 10.1002/1873-3468.12677. Epub 2017, Jun 14. PMID:28504306 doi:http://dx.doi.org/10.1002/1873-3468.12677
- ↑ 2.0 2.1 Seven AB, Barros-Alvarez X, de Lapeyriere M, Papasergi-Scott MM, Robertson MJ, Zhang C, Nwokonko RM, Gao Y, Meyerowitz JG, Rocher JP, Schelshorn D, Kobilka BK, Mathiesen JM, Skiniotis G. G-protein activation by a metabotropic glutamate receptor. Nature. 2021 Jun 30. pii: 10.1038/s41586-021-03680-3. doi:, 10.1038/s41586-021-03680-3. PMID:34194039 doi:http://dx.doi.org/10.1038/s41586-021-03680-3
Student Contributors
- Courtney Vennekotter
- Cade Chezem