We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1711
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
=== Conformation: Closed === | === Conformation: Closed === | ||
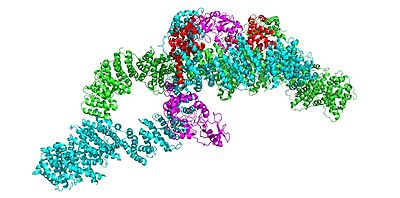
| - | In the overall | + | In the overall <scene name='90/905640/Closedoverall/1'>closed neurofibromin</scene> (and inactive) conformation, both sets of the GRD and Sec14-PH domains are rotated in a way that they are inaccessible and inactive. The closed conformation has both of the protomers/chains in the closed positions, whereas the open conformation has one closed and one open protomer. You can see that in the closed conformation, Ras binding by the GRD domain is sterically hindered and there is no room for association with the Ras protein. |
The closed conformation has the GRD and Sec14-PH domains oriented in a way that the amino acids C1032, H1558, and H1576 are in close proximity to each other to form a transition metal-binding site with zinc. The fourth coordination partner in this is water. | The closed conformation has the GRD and Sec14-PH domains oriented in a way that the amino acids C1032, H1558, and H1576 are in close proximity to each other to form a transition metal-binding site with zinc. The fourth coordination partner in this is water. | ||
<scene name='90/905640/Closedoverall/6'>vertical view</scene> | <scene name='90/905640/Closedoverall/6'>vertical view</scene> | ||
| Line 23: | Line 23: | ||
The <scene name='90/905640/Grd_domains/2'>GRD domain</scene> of Neurofibromin, specifically the arginine finger (R1276), binds to the Ras + GTP complex. | The <scene name='90/905640/Grd_domains/2'>GRD domain</scene> of Neurofibromin, specifically the arginine finger (R1276), binds to the Ras + GTP complex. | ||
=== Key Players === | === Key Players === | ||
| - | + | An <scene name='90/905640/Arg_finger/1'>R1276</scene> (R1276) present in the GRD is critical for Ras binding and is only accessible when the GRD and Sec14-PH domains are rotated in such a way that there is no steric hindrance from the surrounding dimer chains. The Closed conformation is stabilized by a triade of residues that are coordinated with transition metal-binding sites with zinc. Here, the GRD and Sec14-PH domains are oriented in a way that the H1558 and H1576 are able to interact with C1032 and form a transition binding-site with zinc. This binding site stabilizes the closed conformation and prevents Ras from associating with the GRD based on the location of the GRD in relation to the rest of the protein. | |
== Function == | == Function == | ||
| - | + | The GRD arginine finger (R1276) is crucial in Ras binding. When Neurofibromin is in the open conformation, the Arginine finger is able to bind with Ras because the Arginine is not inhibited as it would be in the closed conformation, where R1276 is facing the core and inaccessible due to the rotation of the two domains. This open conformation allows neurofibromin to associate with Ras via R1276 binding, which then hydrolyzes the Ras from active GTP to inactive GDP, showing why R1276 is crucial to neurofibromin function. In the open conformation, on the other hand, the arginine finger is able to interact with Ras and hydrolyze it which effectively turns it off. | |
=== Ras Control === | === Ras Control === | ||
Ras is still promoting cell proliferation in this closed conformation because Neurofibromin is unable to hydrolyze Ras and inactivate it. In this open conformation, the Ras is not sterically hindered and the Arginine finger is accessible for Ras binding, thus allowing Neurofibromin to down-regulate Ras. | Ras is still promoting cell proliferation in this closed conformation because Neurofibromin is unable to hydrolyze Ras and inactivate it. In this open conformation, the Ras is not sterically hindered and the Arginine finger is accessible for Ras binding, thus allowing Neurofibromin to down-regulate Ras. | ||
Revision as of 19:26, 28 March 2022
Neurofibromin
| |||||||||||
References
- ↑ Bergoug M, Doudeau M, Godin F, Mosrin C, Vallee B, Benedetti H. Neurofibromin Structure, Functions and Regulation. Cells. 2020 Oct 27;9(11). pii: cells9112365. doi: 10.3390/cells9112365. PMID:33121128 doi:http://dx.doi.org/10.3390/cells9112365
- ↑ Ransey E, Paredes E, Dey SK, Das SR, Heroux A, Macbeth MR. Crystal structure of the Entamoeba histolytica RNA lariat debranching enzyme EhDbr1 reveals a catalytic Zn(2+) /Mn(2+) heterobinucleation. FEBS Lett. 2017 Jul;591(13):2003-2010. doi: 10.1002/1873-3468.12677. Epub 2017, Jun 14. PMID:28504306 doi:http://dx.doi.org/10.1002/1873-3468.12677
Student Contributors
- Hannah Luchinski