We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1703
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
===Inactive State=== | ===Inactive State=== | ||
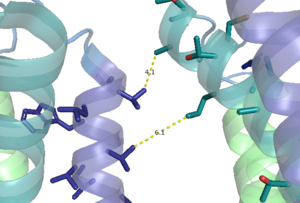
| - | A few hallmarks of the inactive structure of mGlu2 are the Venus FlyTrap Domain in the open conformation, well separated Cysteine-Rich Domains, and distinct orientation of the 7 Transmembrane Domains (7TM). Perhaps the most critical component of the inactive form is the asymmetric TM3-TM4 interface formed by both of the 7 alpha helices in the alpha and beta chains in the transmembrane domain. The transmembrane domain is mediated mainly by helix IV on the alpha chain and helix lll on the beta chain of the dimer through hydrophobic interactions. These hydrophobic interactions between both transmembrane helices stabilize inactive conformation of mGlu2<ref name="Lin"/>. | + | A few hallmarks of the inactive structure of mGlu2 are the Venus FlyTrap Domain in the open conformation, well separated Cysteine-Rich Domains, and distinct orientation of the 7 Transmembrane Domains (7TM). Perhaps the most critical component of the inactive form is the <scene name='90/904307/Tmd_helices/3'>asymmetric TM3-TM4 interface</scene> formed by both of the 7 alpha helices in the alpha and beta chains in the transmembrane domain. The transmembrane domain is mediated mainly by helix IV on the alpha chain and helix lll on the beta chain of the dimer through hydrophobic interactions. These hydrophobic interactions between both transmembrane helices stabilize inactive conformation of mGlu2<ref name="Lin"/>. |
| - | + | [[Image:TM4_hydrophobic_interactions.png|300 px|left|thumb|'''Figure 2.''' Hydrophobic interactions of transmembrane helices III and IV that stabilize the inactive form of mGlu2.]] | |
===Intermediate Form=== | ===Intermediate Form=== | ||
Although there are no Cryo-EM images of the intermediate form, it is still a very important state that mGlu2 goes through. The <scene name='90/904308/Agonist_binding_site/4'>Agonist Binding Site</scene> is formed by both lobes of the Venus FlyTrap Domain. The receptor will remain in this inactive state if there are insufficient concentrations of glutamate available<ref name="Du" />. Since glutamate is the main excitatory neurotransmitter in the central nervous system, its ability to bind is extremely important, especially for cell excitability. | Although there are no Cryo-EM images of the intermediate form, it is still a very important state that mGlu2 goes through. The <scene name='90/904308/Agonist_binding_site/4'>Agonist Binding Site</scene> is formed by both lobes of the Venus FlyTrap Domain. The receptor will remain in this inactive state if there are insufficient concentrations of glutamate available<ref name="Du" />. Since glutamate is the main excitatory neurotransmitter in the central nervous system, its ability to bind is extremely important, especially for cell excitability. | ||
Revision as of 01:09, 29 March 2022
==Metabotropic Glutamate Receptor 2==
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Lin S, Han S, Cai X, Tan Q, Zhou K, Wang D, Wang X, Du J, Yi C, Chu X, Dai A, Zhou Y, Chen Y, Zhou Y, Liu H, Liu J, Yang D, Wang MW, Zhao Q, Wu B. Structures of Gi-bound metabotropic glutamate receptors mGlu2 and mGlu4. Nature. 2021 Jun;594(7864):583-588. doi: 10.1038/s41586-021-03495-2. Epub 2021, Jun 16. PMID:34135510 doi:http://dx.doi.org/10.1038/s41586-021-03495-2
- ↑ Seven, Alpay B., et al. “G-Protein Activation by a Metabotropic Glutamate Receptor.” Nature News, Nature Publishing Group, 30 June 2021, https://www.nature.com/articles/s1586-021-03680-3
- ↑ Zhang, Zhu, et al. “Roles of Glutamate Receptors in Parkinson's Disease.” MDPI, Multidisciplinary Digital Publishing Institute, 6 Sept. 2019, https://dx.doi.org/10.3390%2Fijms20184391.>
- ↑ Yang, Hong-Ju, et al. “Deletion of Type 2 Metabotropic Glutamate Receptor Decreases Sensitivity to Cocaine Reward in Rats.” Cell Reports, U.S. National Library of Medicine, 11 July 2017, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5555082/.>
- ↑ 5.0 5.1 Du, Juan, et al. “Structures of Human mglu2 and mglu7 Homo- and Heterodimers.” Nature News, Nature Publishing Group, 16 June 2021, https://www.nature.com/articles/s41586-021-03641-w.>
Student Contributors
Frannie Brewer and Ashley Wilkinson