Sandbox Reserved 1709
From Proteopedia
| Line 16: | Line 16: | ||
=== Cap Domain === | === Cap Domain === | ||
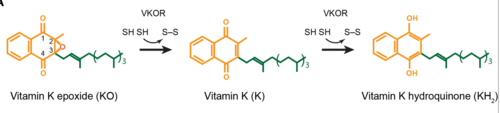
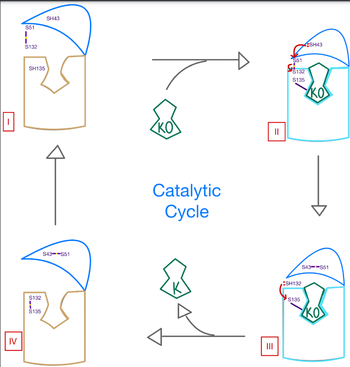
| - | A key part of VKOR is the function of the <scene name='90/904314/Cap_domain/3'>Cap Domain</scene>, which is located right above the helices of VKOR towards the intracellular part of the membrane. The cap is in a helical shape and is located in close proximity to the Anchor and beta hairpin to maintain in the proper orientation. The cap domain assists with activating Vitamin K as it induces the structural change of VKOR from the open conformation to the closed conformation when the substrate binds. This initiates a domino effect through the [https://reader.elsevier.com/reader/sd/pii/S0021925820001386?token=9F8E1964241D20488CA55E035D35D9A5D650A7B3FDAD9A5579598A8DC00127539BE71CF1785B117102144AC1F41ABB6C&originRegion=us-east-1&originCreation=20220329001707/ catalytic mechanism]. The cap domain has critical interactions that stabilize the closed conformation including a <scene name='90/904314/Disulfide_bridge_stabilization/ | + | A key part of VKOR is the function of the <scene name='90/904314/Cap_domain/3'>Cap Domain</scene>, which is located right above the helices of VKOR towards the intracellular part of the membrane. The cap is in a helical shape and is located in close proximity to the Anchor and beta hairpin to maintain in the proper orientation. The cap domain assists with activating Vitamin K as it induces the structural change of VKOR from the open conformation to the closed conformation when the substrate binds. This initiates a domino effect through the [https://reader.elsevier.com/reader/sd/pii/S0021925820001386?token=9F8E1964241D20488CA55E035D35D9A5D650A7B3FDAD9A5579598A8DC00127539BE71CF1785B117102144AC1F41ABB6C&originRegion=us-east-1&originCreation=20220329001707/ catalytic mechanism]. The cap domain has critical interactions that stabilize the closed conformation including a <scene name='90/904314/Disulfide_bridge_stabilization/3'>Disulfide Bridge</scene> between S43 and S51, and polar interactions from D44. |
=== Anchor === | === Anchor === | ||
Revision as of 13:54, 29 March 2022
| |||||||||||
References
1. Elshaikh, A. O., Shah, L., Joy Mathew, C., Lee, R., Jose, M. T., & Cancarevic, I. "Influence of Vitamin K on Bone Mineral Density and Osteoporosis" (2020) Cureus, 12(10), e10816. [1]
2. Guomin Shen, Weidong Cui, Qing Cao, Meng Gao, Hongli Liu, Gaigai Su, Michael L. Gross, Weikai Li. The catalytic mechanism of vitamin K epoxide reduction in a cellular environment. (2021) Journal of Biological Chemistry, Volume 296,100145. https://doi.org/10.1074/jbc.RA120.015401.
3. Li, Weikai et al. “Structure of a bacterial homologue of vitamin K epoxide reductase.” Nature vol. 463,7280 (2010): 507-12. doi:10.1038/nature08720.
4. Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2021 Jan 1;371(6524):eabc5667. doi: 10.1126/science.abc5667. Epub 2020 Nov 5. PMID: 33154105; PMCID: PMC7946407.
5. “Warfarin.” Wikipedia, Wikimedia Foundation, 10 Feb. 2022, https://en.wikipedia.org/wiki/Warfarin.
6. Yang W., et. al. “VKORC1 Haplotypes Are Associated With Arterial Vascular Diseases (Stroke, Coronary Heart Disease, and Aortic Dissection)” (2006) Circulation. ;113:1615–1621 [2]