Sandbox Reserved 1709
From Proteopedia
| Line 23: | Line 23: | ||
=== Brief Overview === | === Brief Overview === | ||
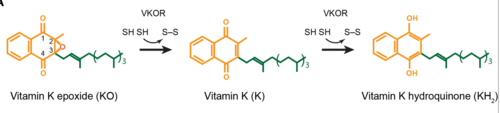
| - | The <scene name='90/906893/Open_conformation/1'>open conformation</scene> will be prepped and waiting for a substrate or ligand to bind. Once the substrate binds, this will induce the <scene name='90/904314/Closed_conformation/1'>Closed conformation</scene> of VKOR, where the catalytic mechanism will activate Vitamin K via reactive cysteine residues. Vitamin K will then be released from the binding pocket once it is fully activated for use in the body, and VKOR will resume the open conformation once again. The enzyme will then reset into its reactive state to prep for another molecule of Vitamin K to bind. | + | The <scene name='90/906893/Open_conformation/1'>open conformation</scene> will be prepped and waiting for a substrate or ligand to bind. Once the substrate binds, this will induce the <scene name='90/904314/Closed_conformation/1'> Closed conformation</scene> of VKOR, where the catalytic mechanism will activate Vitamin K via reactive cysteine residues. Vitamin K will then be released from the binding pocket once it is fully activated for use in the body, and VKOR will resume the open conformation once again. The enzyme will then reset into its reactive state to prep for another molecule of Vitamin K to bind. |
| Line 29: | Line 29: | ||
[[Image:Catalytic Mech Pic.png |350 px| right| thumb]] | [[Image:Catalytic Mech Pic.png |350 px| right| thumb]] | ||
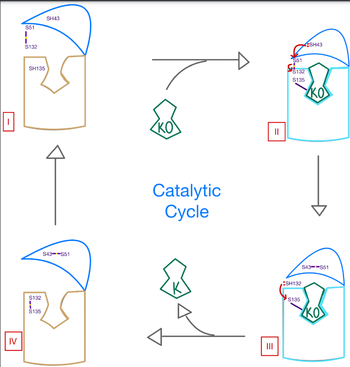
| - | The catalytic mechanism of VKOR is a critical part of its overall function in the body. Highly regulated enzymatic activity through the reactivity of catalytic cysteines allows VKOR to properly activate Vitamin K for its use in the body. The enzyme begins in <scene name='90/906893/Stage_1_catalytic_cycle/1'>stage 1</scene>, where it's in the open conformation with the cap domain open to allow in a substrate to bind to the active site. Once a substrate binds, the cap domain is initiated into the closed conformation. VKOR is now in <scene name='90/906893/Stage_2_catalytic_cycle/1'>stage 2</scene>. To further stabilize the closed conformation with the substrate bound, the cap domain helps initiate a catalytic reaction of cysteines to break the disulfide bridge that was stabilizing stage 1. Free cysteines are now available that provide strong stabilization of the closed conformation through interactions with the cap domain and the bound substrate. This puts the enzyme in <scene name='90/904314/Stage_3_catalytic_cycle/2'>stage 3</scene>, where the catalytic free cysteines react to form a new disulfide bridge, releasing the activated substrate into the blood stream to promote anticoagulation. With two stable disulfide bridges and VKOR unbound, the enzyme is now in its final, unreactive <scene name='90/904314/Stage_4_catalytic_cycle/ | + | The catalytic mechanism of VKOR is a critical part of its overall function in the body. Highly regulated enzymatic activity through the reactivity of catalytic cysteines allows VKOR to properly activate Vitamin K for its use in the body. The enzyme begins in <scene name='90/906893/Stage_1_catalytic_cycle/1'>stage 1</scene>, where it's in the open conformation with the cap domain open to allow in a substrate to bind to the active site. Once a substrate binds, the cap domain is initiated into the closed conformation. VKOR is now in <scene name='90/906893/Stage_2_catalytic_cycle/1'>stage 2</scene>. To further stabilize the closed conformation with the substrate bound, the cap domain helps initiate a catalytic reaction of cysteines to break the disulfide bridge that was stabilizing stage 1. Free cysteines are now available that provide strong stabilization of the closed conformation through interactions with the cap domain and the bound substrate. This puts the enzyme in <scene name='90/904314/Stage_3_catalytic_cycle/2'>stage 3</scene>, where the catalytic free cysteines react to form a new disulfide bridge, releasing the activated substrate into the blood stream to promote anticoagulation. With two stable disulfide bridges and VKOR unbound, the enzyme is now in its final, unreactive <scene name='90/904314/Stage_4_catalytic_cycle/4'>stage 4</scene>. VKOR must undergo conformational changes to return to Stage 1 and restart the catalytic process to activate Vitamin K again. |
== Disease and Treatment == | == Disease and Treatment == | ||
Revision as of 14:01, 29 March 2022
| |||||||||||
References
1. Elshaikh, A. O., Shah, L., Joy Mathew, C., Lee, R., Jose, M. T., & Cancarevic, I. "Influence of Vitamin K on Bone Mineral Density and Osteoporosis" (2020) Cureus, 12(10), e10816. [1]
2. Guomin Shen, Weidong Cui, Qing Cao, Meng Gao, Hongli Liu, Gaigai Su, Michael L. Gross, Weikai Li. The catalytic mechanism of vitamin K epoxide reduction in a cellular environment. (2021) Journal of Biological Chemistry, Volume 296,100145. https://doi.org/10.1074/jbc.RA120.015401.
3. Li, Weikai et al. “Structure of a bacterial homologue of vitamin K epoxide reductase.” Nature vol. 463,7280 (2010): 507-12. doi:10.1038/nature08720.
4. Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2021 Jan 1;371(6524):eabc5667. doi: 10.1126/science.abc5667. Epub 2020 Nov 5. PMID: 33154105; PMCID: PMC7946407.
5. “Warfarin.” Wikipedia, Wikimedia Foundation, 10 Feb. 2022, https://en.wikipedia.org/wiki/Warfarin.
6. Yang W., et. al. “VKORC1 Haplotypes Are Associated With Arterial Vascular Diseases (Stroke, Coronary Heart Disease, and Aortic Dissection)” (2006) Circulation. ;113:1615–1621 [2]