Sandbox Reserved 1709
From Proteopedia
| Line 16: | Line 16: | ||
=== Cap Domain === | === Cap Domain === | ||
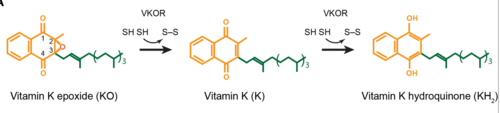
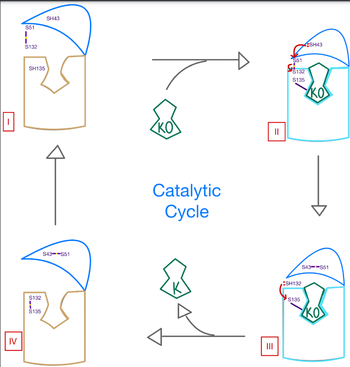
| - | A key part of VKOR is the function of the <scene name='90/904314/ cap_domain/3'>Cap Domain</scene>, which is located right above the helices of VKOR towards the intracellular part of the membrane. The cap is in a helical shape and is located in close proximity to the Anchor and beta hairpin to maintain in the proper orientation. The cap domain assists with activating Vitamin K as it induces the structural change of VKOR from the open conformation to the closed conformation when the substrate binds. This initiates a domino effect through the [https://reader.elsevier.com/reader/sd/pii/S0021925820001386?token=9F8E1964241D20488CA55E035D35D9A5D650A7B3FDAD9A5579598A8DC00127539BE71CF1785B117102144AC1F41ABB6C&originRegion=us-east-1&originCreation=20220329001707/ catalytic mechanism]. The cap domain has critical interactions that stabilize the closed conformation including a <scene name='90/904314/Disulfide_bridge_stabilization/3'> disulfide | + | A key part of VKOR is the function of the <scene name='90/904314/ cap_domain/3'>Cap Domain</scene>, which is located right above the helices of VKOR towards the intracellular part of the membrane. The cap is in a helical shape and is located in close proximity to the Anchor and beta hairpin to maintain in the proper orientation. The cap domain assists with activating Vitamin K as it induces the structural change of VKOR from the open conformation to the closed conformation when the substrate binds. This initiates a domino effect through the [https://reader.elsevier.com/reader/sd/pii/S0021925820001386?token=9F8E1964241D20488CA55E035D35D9A5D650A7B3FDAD9A5579598A8DC00127539BE71CF1785B117102144AC1F41ABB6C&originRegion=us-east-1&originCreation=20220329001707/ catalytic mechanism]. The cap domain has critical interactions that stabilize the closed conformation including a <scene name='90/904314/Disulfide_bridge_stabilization/3'> disulfide bridge</scene> between S43 and S51, and polar interactions from D44. |
=== Anchor === | === Anchor === | ||
| - | The <scene name='90/904314/Anchor_domain/3'> anchor | + | The <scene name='90/904314/Anchor_domain/3'> anchor domain</scene> sticks out from the side of VKOR with the primary role of stabilizing the enzyme within the membrane. To accomplish this, hydrophilic and hydrophobic residues are highly conserved with hydrophilic residues located to interact with the outer hydrophilic leaflet of the bilipid membrane, while the hydrophobic residues on the anchor have strong interactions with the inner hydrophobic leaflet of the bilipid membrane. These sufficient interactions allow for VKOR to remain in the proper arrangement and proximity within the membrane for Vitamin K to properly bind to be activated to achieve its biological function. |
== Function: Method of Coagulation == | == Function: Method of Coagulation == | ||
| Line 35: | Line 35: | ||
Since activated Vitamin K plays a crucial role in blood coagulation in the body, any defects in the function and enzymatic activity of VKOR may have detrimental effects on Vitamin K's ability to promote important blood clotting. Ultimately, mutations in VKOR may lead to increased susceptibility to vascular diseases, such as a stroke [https://doi.org/10.1161/CIRCULATIONAHA.105.580167]. Vitamin K has also been shown to have an important role in maintaining bone health, so inactivity of VKOR could also be linked to decreased bone density and osteoporosis [https://doi.org/10.7759/cureus.10816]. | Since activated Vitamin K plays a crucial role in blood coagulation in the body, any defects in the function and enzymatic activity of VKOR may have detrimental effects on Vitamin K's ability to promote important blood clotting. Ultimately, mutations in VKOR may lead to increased susceptibility to vascular diseases, such as a stroke [https://doi.org/10.1161/CIRCULATIONAHA.105.580167]. Vitamin K has also been shown to have an important role in maintaining bone health, so inactivity of VKOR could also be linked to decreased bone density and osteoporosis [https://doi.org/10.7759/cureus.10816]. | ||
=== Inhibition === | === Inhibition === | ||
| - | The most inexpensive and common way to treat blood clotting is through the VKOR inhibitor, <scene name='90/906893/Vkor_with_warfarin_bound/1'> Warfarin</scene>. [https://en.wikipedia.org/wiki/Warfarin Warfarin] is able to do so by outcompeting KO, such that Vitamin K cannot be activated to promote coagulation in the blood. Warfarin will enter the binding pocket of VKOR, creating strong hydrogen bonds with the active site. Warfarin resistance may also occur, decreasing the effective anticoagulation some drugs may be attempting to promote. | + | The most inexpensive and common way to treat blood clotting is through the VKOR inhibitor, <scene name='90/906893/Vkor_with_warfarin_bound/1'> Warfarin</scene>. [https://en.wikipedia.org/wiki/Warfarin Warfarin] is able to do so by outcompeting KO, such that Vitamin K cannot be activated to promote coagulation in the blood. Warfarin will enter the binding pocket of VKOR, creating strong hydrogen bonds with the active site. Warfarin resistance may also occur due to mutations of VKOR, decreasing the effective anticoagulation some drugs may be attempting to promote. The degree of resistance is important to determine so that warfarin may be an effective anticoagulant without being detrimentally effective in blood flow. |
=== Mutations === | === Mutations === | ||
Revision as of 14:37, 29 March 2022
| |||||||||||
References
1. Elshaikh, A. O., Shah, L., Joy Mathew, C., Lee, R., Jose, M. T., & Cancarevic, I. "Influence of Vitamin K on Bone Mineral Density and Osteoporosis" (2020) Cureus, 12(10), e10816. [1]
2. Guomin Shen, Weidong Cui, Qing Cao, Meng Gao, Hongli Liu, Gaigai Su, Michael L. Gross, Weikai Li. The catalytic mechanism of vitamin K epoxide reduction in a cellular environment. (2021) Journal of Biological Chemistry, Volume 296,100145. https://doi.org/10.1074/jbc.RA120.015401.
3. Li, Weikai et al. “Structure of a bacterial homologue of vitamin K epoxide reductase.” Nature vol. 463,7280 (2010): 507-12. doi:10.1038/nature08720.
4. Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2021 Jan 1;371(6524):eabc5667. doi: 10.1126/science.abc5667. Epub 2020 Nov 5. PMID: 33154105; PMCID: PMC7946407.
5. Yang W., et. al. “VKORC1 Haplotypes Are Associated With Arterial Vascular Diseases (Stroke, Coronary Heart Disease, and Aortic Dissection)” (2006) Circulation. ;113:1615–1621 [2]