Sandbox Reserved 1716
From Proteopedia
(Difference between revisions)
| Line 50: | Line 50: | ||
==Significant Cysteines== | ==Significant Cysteines== | ||
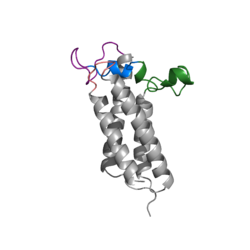
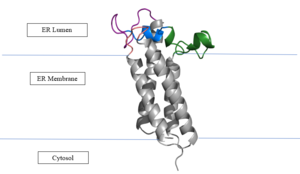
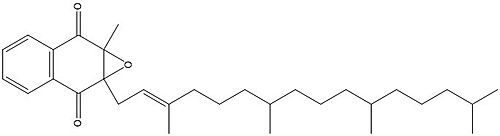
| - | A set of four cysteines is consistently conserved in all VKOR homologs. In the human homolog (HsVKOR) these cysteines are Cys43, Cys51, Cys132, and Cys 135. <scene name='90/904321/Cysteines/6'>Significant Cysteines</scene> In the Pufferfish homolog (TrVKORL) these cysteines, due to Cryo-EM differences,are Cys52, Cys55, Cys141, and Cys144. These cysteines are the key factor that allow for Vitamin K Epoxide Reductase to perform its function, which is to open the epoxide ring on Vitamin K Epoxide in order to re-make Vitamin K Quinone. In the closed conformation, that is induced when Vitamin K binds in the hydrophobic pocket, Cys-132 binds to Cys-51 and Cys-135 will bind to the 3' hydroxyl group on Vitamin K Epoxide, which allows for the electron transfer to open up the epoxide ring. <scene name='90/904321/Cys52disulfidecys55/ | + | A set of four cysteines is consistently conserved in all VKOR homologs. In the human homolog (HsVKOR) these cysteines are Cys43, Cys51, Cys132, and Cys 135. <scene name='90/904321/Cysteines/6'>Significant Cysteines</scene> In the Pufferfish homolog (TrVKORL) these cysteines, due to Cryo-EM differences,are Cys52, Cys55, Cys141, and Cys144. These cysteines are the key factor that allow for Vitamin K Epoxide Reductase to perform its function, which is to open the epoxide ring on Vitamin K Epoxide in order to re-make Vitamin K Quinone. In the closed conformation, that is induced when Vitamin K binds in the hydrophobic pocket, Cys-132 binds to Cys-51 and Cys-135 will bind to the 3' hydroxyl group on Vitamin K Epoxide, which allows for the electron transfer to open up the epoxide ring. <scene name='90/904321/Cys52disulfidecys55/9'>Electron Transfer through Cysteine138 TrVKORL</scene> |
Revision as of 19:48, 29 March 2022
Vitamin K Epoxide Reductase
| |||||||||||
References
- ↑ Ransey E, Paredes E, Dey SK, Das SR, Heroux A, Macbeth MR. Crystal structure of the Entamoeba histolytica RNA lariat debranching enzyme EhDbr1 reveals a catalytic Zn(2+) /Mn(2+) heterobinucleation. FEBS Lett. 2017 Jul;591(13):2003-2010. doi: 10.1002/1873-3468.12677. Epub 2017, Jun 14. PMID:28504306 doi:http://dx.doi.org/10.1002/1873-3468.12677
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ Olson RE. The function and metabolism of vitamin K. Annu Rev Nutr. 1984;4:281-337. doi: 10.1146/annurev.nu.04.070184.001433. PMID:6380538 doi:http://dx.doi.org/10.1146/annurev.nu.04.070184.001433
- ↑ Wu S, Chen X, Jin DY, Stafford DW, Pedersen LG, Tie JK. Warfarin and vitamin K epoxide reductase: a molecular accounting for observed inhibition. Blood. 2018 Aug 9;132(6):647-657. doi: 10.1182/blood-2018-01-830901. Epub 2018, May 9. PMID:29743176 doi:http://dx.doi.org/10.1182/blood-2018-01-830901
- ↑ Chatron N, Abi Khalil R, Benoit E, Lattard V. Structural Investigation of the Vitamin K Epoxide Reductase (VKORC1) Binding Site with Vitamin K. Biochemistry. 2020 Apr 7;59(13):1351-1360. doi: 10.1021/acs.biochem.9b01084. Epub, 2020 Mar 23. PMID:32182040 doi:http://dx.doi.org/10.1021/acs.biochem.9b01084
- ↑ Shen G, Cui W, Cao Q, Gao M, Liu H, Su G, Gross ML, Li W. The catalytic mechanism of vitamin K epoxide reduction in a cellular environment. J Biol Chem. 2021 Jan-Jun;296:100145. doi: 10.1074/jbc.RA120.015401. Epub 2020, Dec 10. PMID:33273012 doi:http://dx.doi.org/10.1074/jbc.RA120.015401