We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1717
From Proteopedia
(Difference between revisions)
| Line 38: | Line 38: | ||
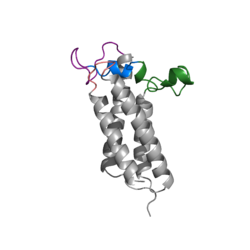
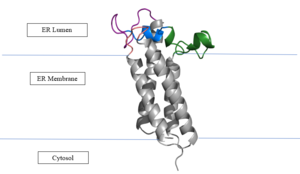
The VKOR enzyme is made up of four transmembrane helices: T1, T2, T3, and T4.(Grey) Each of these helices come together to form a central pocket, that is topped by a cap domain. In the cap domain are important regions that are significant for Vitamin K binding, and the overall function of Vitamin K Epoxide Reductase. These important regions are the Anchor(Green), Cap Region (Blue), Beta Hairpin (Purple), and 3-4 Loop (Pink). The transmembrane helices form the central pocket that is also the active site of the enzyme. This is because the catalytic cysteines Cys132 and Cys135 are located in this region of the enzyme. | The VKOR enzyme is made up of four transmembrane helices: T1, T2, T3, and T4.(Grey) Each of these helices come together to form a central pocket, that is topped by a cap domain. In the cap domain are important regions that are significant for Vitamin K binding, and the overall function of Vitamin K Epoxide Reductase. These important regions are the Anchor(Green), Cap Region (Blue), Beta Hairpin (Purple), and 3-4 Loop (Pink). The transmembrane helices form the central pocket that is also the active site of the enzyme. This is because the catalytic cysteines Cys132 and Cys135 are located in this region of the enzyme. | ||
| - | The transmembrane helices make up the ER-luminal region, which is large and flexible. Vitamin K Epoxide Reductase is known for its in-vitro instability. When trying to view the structure an extra protein known as sfGFP, superfolder green flourescent protein, is bound the N and C termini of Vitamin K Epoxide. For the purpose of viewing the structure, this protein has been removed from the pdb files. | + | The transmembrane helices make up the ER-luminal region, which is large and flexible. Vitamin K Epoxide Reductase is known for its in-vitro instability. When trying to view the structure an extra protein known as sfGFP, superfolder green flourescent protein, is bound the N and C termini of Vitamin K Epoxide. For the purpose of viewing the structure, this protein has been removed from the pdb files. |
| + | <ref name="Ransey">PMID:28504306</ref> | ||
===Transmembrane Helices=== | ===Transmembrane Helices=== | ||
The Transmembrane helices are named Transmembrane Helix 1, Transmembrane Helix 2, Transmembrane Helix 3, and Transmembrane Helix 4. The residues on Transmembrane Helix 2 (TM2) and Transmembrane Helix 4 (TM4) are significant for the binding of Vitamin K to the hydrophobic pocket of the enzyme. Asparagine 83 on TM2 and Tyrosine 142 hydrogen bond to Vitamin K Epoxide, in order to hold it in place so that it may be reduced. The angle in which Vitamin K Epoxide binds is significant to the placement of the beta hairpin, and loop 3-4. Cysteine residues from the beta hairpin and loop 3-4 will donate their electrons to Vitamin K Epoxide to open the epoxide ring, and reform Vitamin K Quinone. | The Transmembrane helices are named Transmembrane Helix 1, Transmembrane Helix 2, Transmembrane Helix 3, and Transmembrane Helix 4. The residues on Transmembrane Helix 2 (TM2) and Transmembrane Helix 4 (TM4) are significant for the binding of Vitamin K to the hydrophobic pocket of the enzyme. Asparagine 83 on TM2 and Tyrosine 142 hydrogen bond to Vitamin K Epoxide, in order to hold it in place so that it may be reduced. The angle in which Vitamin K Epoxide binds is significant to the placement of the beta hairpin, and loop 3-4. Cysteine residues from the beta hairpin and loop 3-4 will donate their electrons to Vitamin K Epoxide to open the epoxide ring, and reform Vitamin K Quinone. | ||
| Line 45: | Line 46: | ||
===Cap Domain=== | ===Cap Domain=== | ||
| - | The cap domain of Vitamin K Epoxide Reductase plays an intricate role in its function. When Vitamin K Epoxide (or a similar substrate) binds in the hydrophobic pocket of VKOR, the cap domain undergoes a conformational change that will allow for specific cysteine residues to be able to open the epoxide ring and to recreate Vitamin K Quinone. The catalytic cycle begins in an open fully oxidized conformation. <scene name='90/904321/Openvkor/ | + | The cap domain of Vitamin K Epoxide Reductase plays an intricate role in its function. When Vitamin K Epoxide (or a similar substrate) binds in the hydrophobic pocket of VKOR, the cap domain undergoes a conformational change that will allow for specific cysteine residues to be able to open the epoxide ring and to recreate Vitamin K Quinone. The catalytic cycle begins in an open fully oxidized conformation. <scene name='90/904321/Openvkor/3'>Open Oxidized Conformation</scene> This conformation has slightly different parts. These include the Anchor (green), the cap region (blue), 3-4 Loop (pink), and luminal helix (yellow). When Vitamin K Epoxide binds, the entire cap domain undergoes a slight conformation change, but the luminal helix has a larger change. The luminal helix (yellow) bends forward where specific cysteines on this region are in proximity to other important cysteines. The luminal helix is then referred to as the beta hairpin (purple). <scene name='90/904321/Vkoclosed/3'>Partially Oxidized Closed Conformation</scene> |
==Significant Cysteines== | ==Significant Cysteines== | ||
| - | A set of four cysteines is consistently conserved in all VKOR homologs. In the human homolog (HsVKOR) these cysteines are Cys43, Cys51, Cys132, and Cys 135. <scene name='90/ | + | A set of four cysteines is consistently conserved in all VKOR homologs. In the human homolog (HsVKOR) these cysteines are Cys43, Cys51, Cys132, and Cys 135. <scene name='90/904321/Cysteines/6'>Significant Cysteines</scene> In the Pufferfish homolog (TrVKORL) these cysteines, due to Cryo-EM differences,are Cys52, Cys55, Cys141, and Cys144. These cysteines are the key factor that allow for Vitamin K Epoxide Reductase to perform its function, which is to open the epoxide ring on Vitamin K Epoxide in order to re-make Vitamin K Quinone. In the closed conformation, that is induced when Vitamin K binds in the hydrophobic pocket, Cys-132 binds to Cys-51 and Cys-135 will bind to the 3' hydroxyl group on Vitamin K Epoxide, which allows for the electron transfer to open up the epoxide ring. <scene name='90/904321/Cys52disulfidecys55/9'>Electron Transfer through Cysteine138 TrVKORL</scene> |
| Line 61: | Line 62: | ||
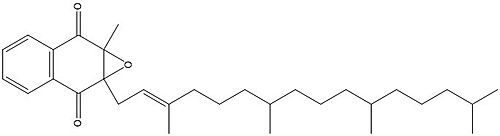
=== Binding === | === Binding === | ||
| - | To start, VKOR is in its <scene name='90/904322/Open_conformation/1'>open conformation</scene>. The Vitamin K epoxide enters. The oxygens of the ketones bind to <scene name='90/904322/Vko_binding/1'>Asn80 and Tyr139</scene>. With Vitamin K epoxide in its place, the conformation of VKOR is partially oxidized in regards to the cysteine pairs, which overall leads to the reduction of the substrate. A disulfide bond forms between Cys51 and Cys132, resulting in the closed conformation. This leaves the sulfur on Cys43 and the sulfur on Cys135 protonated. The available hydrogens on these cysteines are utilized in reducing the epoxide. First, the sulfur on Cys51 and Cys43 form a new bond. The hydrogen from Cys43 binds to the oxygen in the epoxide. The sulfur on Cys132 and the sulfur on Cys135 then form a new disulfide bond. The hydrogen that was present on Cys135 forms a new bond with the oxygen of the epoxide. With these cysteine pairs formed, VKOR is left in an open conformation. The end products are the Vitamin K/quinone and water. | + | To start, VKOR is in its <scene name='90/904322/Open_conformation/1'>open conformation</scene>. The Vitamin K epoxide enters through the isoprenyl- chain tunnel. The oxygens of the ketones bind to <scene name='90/904322/Vko_binding/1'>Asn80 and Tyr139</scene>. With Vitamin K epoxide in its place, the conformation of VKOR is partially oxidized in regards to the cysteine pairs, which overall leads to the reduction of the substrate. A disulfide bond forms between Cys51 and Cys132, resulting in the closed conformation. This leaves the sulfur on Cys43 and the sulfur on Cys135 protonated. The available hydrogens on these cysteines are utilized in reducing the epoxide. First, the sulfur on Cys51 and Cys43 form a new bond. The hydrogen from Cys43 binds to the oxygen in the epoxide. The sulfur on Cys132 and the sulfur on Cys135 then form a new disulfide bond. The hydrogen that was present on Cys135 forms a new bond with the oxygen of the epoxide. With these cysteine pairs formed, VKOR is left in an open conformation. The end products are the Vitamin K/quinone and water. |
Revision as of 20:02, 29 March 2022
Vitamin K Epoxide Reductase
| |||||||||||