Sandbox Reserved 1716
From Proteopedia
(Difference between revisions)
| Line 54: | Line 54: | ||
| - | ==Vitamin K Epoxide== | + | == Vitamin K Epoxide == |
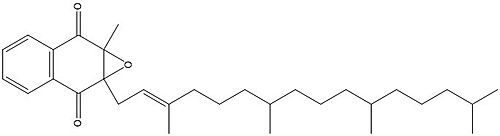
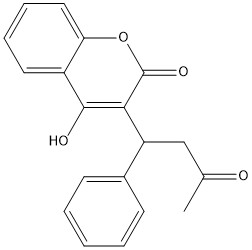
[[Image:Vitamin K epoxide.jpg|500 px|right|thumb|Figure 4. Vitamin K Epoxide structure]] | [[Image:Vitamin K epoxide.jpg|500 px|right|thumb|Figure 4. Vitamin K Epoxide structure]] | ||
| - | |||
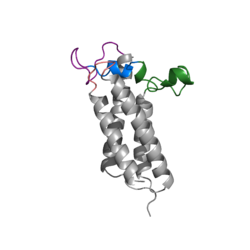
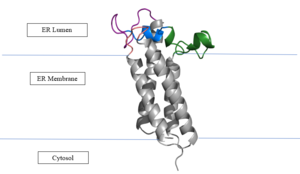
As mentioned above, Vitamin K epoxide is a part of the Vitamin K cycle, necessary for blood coagulation. In the cycle, Vitamin K epoxide reductase (VKOR) reduces Vitamin K epoxide to quinone, or the active form of Vitamin K. What is occurring is VKOR donated electrons to Vitamin K epoxide, and those electrons come from the S-H of one of the cysteine pairs discussed above. The one cysteine pair has to be reduced for the transfer of electrons to the substrate can occur. | As mentioned above, Vitamin K epoxide is a part of the Vitamin K cycle, necessary for blood coagulation. In the cycle, Vitamin K epoxide reductase (VKOR) reduces Vitamin K epoxide to quinone, or the active form of Vitamin K. What is occurring is VKOR donated electrons to Vitamin K epoxide, and those electrons come from the S-H of one of the cysteine pairs discussed above. The one cysteine pair has to be reduced for the transfer of electrons to the substrate can occur. | ||
| - | |||
| - | |||
=== Binding === | === Binding === | ||
| - | To start, VKOR is in its open conformation. The Vitamin K epoxide enters | + | To start, VKOR is in its <scene name='90/904322/Open_conformation/1'>open conformation</scene>. The Vitamin K epoxide enters through the isoprenyl- chain tunnel. The oxygens of the ketones bind to <scene name='90/904322/Vko_binding/1'>Asn80 and Tyr139</scene>. With Vitamin K epoxide in its place, the conformation of VKOR is partially oxidized in regards to the cysteine pairs, which overall leads to the reduction of the substrate. A disulfide bond forms between Cys51 and Cys132, resulting in the closed conformation. This leaves the sulfur on Cys43 and the sulfur on Cys135 protonated. The available hydrogens on these cysteines are utilized in reducing the epoxide. First, the sulfur on Cys51 and Cys43 form a new bond. The hydrogen from Cys43 binds to the oxygen in the epoxide. The sulfur on Cys132 and the sulfur on Cys135 then form a new disulfide bond. The hydrogen that was present on Cys135 forms a new bond with the oxygen of the epoxide. With these cysteine pairs formed, VKOR is left in an open conformation. The end products are the Vitamin K/quinone and water. |
| + | |||
| - | <scene name='90/904321/Vkoh_bonds/1'>Vitamin K Binding</scene> | ||
== Warfarin == | == Warfarin == | ||
[https://en.wikipedia.org/wiki/Warfarin Warfarin] is the most common [https://en.wikipedia.org/wiki/Vitamin_K_antagonist Vitamin K antagonist (VKA)]. Warfarin is a competitive inhibitor, taking the place of Vitamin K Epoxide (VKO) in the active site of Vitamin K Epoxide Reductase (VKOR). When warfarin binds in the active site, it causes VKOR to go into the closed conformation. | [https://en.wikipedia.org/wiki/Warfarin Warfarin] is the most common [https://en.wikipedia.org/wiki/Vitamin_K_antagonist Vitamin K antagonist (VKA)]. Warfarin is a competitive inhibitor, taking the place of Vitamin K Epoxide (VKO) in the active site of Vitamin K Epoxide Reductase (VKOR). When warfarin binds in the active site, it causes VKOR to go into the closed conformation. | ||
| - | [[Image:warfarin.jpg|400 px|right|thumb|Figure | + | [[Image:warfarin.jpg|400 px|right|thumb|Figure 5. 2-Dimensional structure of Warfarin]] |
=== Binding === | === Binding === | ||
| Line 84: | Line 81: | ||
| - | </StructureSection> | ||
== References == | == References == | ||
| + | |||
| + | <ref name="Goodstadt">PMID:15276181</ref> Goodstadt, L., & Ponting, C. P. (2004). Vitamin K epoxide reductase: homology, active site and catalytic mechanism. ''Trends in biochemical sciences, 29''(6), 289–292. https://doi.org/10.1016/j.tibs.2004.04.004 | ||
| + | |||
| + | Rishavy, M.A., Usubalieva, A., Hallgren, K.W., & Berkner, K.L. (2011). Novel insidht into the mechanism of the vitamin K oxidoreductas (VKOR): Electron relay through Cy43 and Cys51 reduces VKOR to allow vitamin K reduction and facilitation of vitamin K-dependent protein caroxylation. ''Journal of Biological Chemistry, 286''(9), 7267-7278. https://doi.org/10.1074/jbc.M110.172213 | ||
| + | |||
| + | <ref name=”Shixuan”>PMID:33154105</ref> Liu, S., Li, S., Shen, G., Sukumar, N., Krezel, A. M., & Li, W. (2021). Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. ''Science (New York, N.Y.), 371''(6524), eabc5667. https://doi.org/10.1126/science.abc5667 | ||
| + | |||
| + | <ref name="Shen">PMID:33273012</ref> Shen, G., Cui, W., Cao, Q., Gao, M., Liu, H., Su, G., Gross, M. L., & Li, W. (2021). The catalytic mechanism of vitamin K epoxide reduction in a cellular environment. ''The Journal of biological chemistry'', 296, 100145. https://doi.org/10.1074/jbc.RA120.015401 | ||
| + | |||
| + | Silverman, R.B. (1981). Chemical model studies for the mechanism of vitamin K epoxide reductase. ''The Journal of American Chemistry Society, 103''(19), 5939-5941. | ||
<ref name="S. Shuang">PMID:33154105</ref> | <ref name="S. Shuang">PMID:33154105</ref> | ||
<ref name="Olson">PMID:6380538</ref> | <ref name="Olson">PMID:6380538</ref> | ||
| Line 91: | Line 97: | ||
<ref name="Chatron">PMID:32182040</ref> | <ref name="Chatron">PMID:32182040</ref> | ||
<ref name="G. Shen">PMID:33273012</ref> | <ref name="G. Shen">PMID:33273012</ref> | ||
| - | <references/> | ||
Revision as of 20:02, 29 March 2022
Vitamin K Epoxide Reductase
| |||||||||||