Neurofibromin
From Proteopedia
(Difference between revisions)
| Line 31: | Line 31: | ||
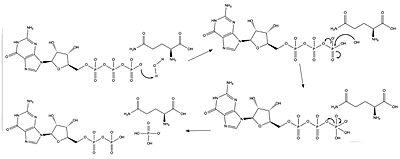
Mutations to the neurofibromin protein are implicated in the progression of Neurofibromatosis type 1 (NF1). This condition drives several forms of human cancers by inactivating the Ras suppression effects of NF, allowing Ras to behave as an oncogene. Neurofibromatosis type 1 is an autosomal dominant disorder that affects 1 in 3,000 people, and the NF gene itself has the highest mutation rate of any known human gene, adding to its prevalence<ref name= ''Lupton''>PMID:34887559</ref>. NF1 primarily causes tumors in the central and peripheral nervous systems, but often has a multisystem expression including tumors in the dermatologic, cardiovascular, gastrointestinal, and orthopedic systems<ref name= ''Cimino''>PMID:29478615</ref>. The wide range of presentations is consistent with the multiplicity of mutations observed in the causative protein<ref name= ''Ly''>PMID:31582003</ref>. | Mutations to the neurofibromin protein are implicated in the progression of Neurofibromatosis type 1 (NF1). This condition drives several forms of human cancers by inactivating the Ras suppression effects of NF, allowing Ras to behave as an oncogene. Neurofibromatosis type 1 is an autosomal dominant disorder that affects 1 in 3,000 people, and the NF gene itself has the highest mutation rate of any known human gene, adding to its prevalence<ref name= ''Lupton''>PMID:34887559</ref>. NF1 primarily causes tumors in the central and peripheral nervous systems, but often has a multisystem expression including tumors in the dermatologic, cardiovascular, gastrointestinal, and orthopedic systems<ref name= ''Cimino''>PMID:29478615</ref>. The wide range of presentations is consistent with the multiplicity of mutations observed in the causative protein<ref name= ''Ly''>PMID:31582003</ref>. | ||
This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
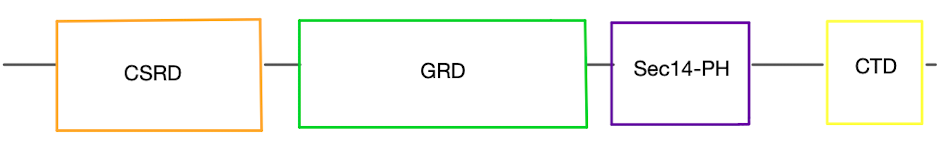
| + | <scene name='90/904326/Open_conformation/2'>Open Conformation</scene>, | ||
| + | <scene name='90/904326/Open_conformation_with_grd_hig/2'>Open Conformation with GRD highlighted</scene> | ||
| + | <scene name='90/904326/Closed_conformation/2'>Closed Conformation</scene>, | ||
| + | <scene name='90/904326/Grd_closed_conformation/2'>Closed Conformation with GRD highlighted</scene> | ||
| + | <scene name='90/904326/Sec15ph_and_grd_open/2'>sec14ph domain in Open Conformation</scene> | ||
| + | <scene name='90/904326/Sec14ph_and_grd_closed/2'>sec14ph domain in Closed Conformation</scene> | ||
| + | <scene name='90/904326/Active_site_with_residues/6'>active site</scene> | ||
</StructureSection> | </StructureSection> | ||
Revision as of 18:32, 31 March 2022
| |||||||||||
References
- ↑ Bergoug M, Doudeau M, Godin F, Mosrin C, Vallee B, Benedetti H. Neurofibromin Structure, Functions and Regulation. Cells. 2020 Oct 27;9(11). pii: cells9112365. doi: 10.3390/cells9112365. PMID:33121128 doi:http://dx.doi.org/10.3390/cells9112365
- ↑ Hall BE, Bar-Sagi D, Nassar N. The structural basis for the transition from Ras-GTP to Ras-GDP. Proc Natl Acad Sci U S A. 2002 Sep 17;99(19):12138-42. Epub 2002 Sep 4. PMID:12213964 doi:http://dx.doi.org/10.1073/pnas.192453199
- ↑ Cimino PJ, Gutmann DH. Neurofibromatosis type 1. Handb Clin Neurol. 2018;148:799-811. doi: 10.1016/B978-0-444-64076-5.00051-X. PMID:29478615 doi:http://dx.doi.org/10.1016/B978-0-444-64076-5.00051-X
- ↑ Scheffzek K, Shivalingaiah G. Ras-Specific GTPase-Activating Proteins-Structures, Mechanisms, and Interactions. Cold Spring Harb Perspect Med. 2019 Mar 1;9(3). pii: cshperspect.a031500. doi:, 10.1101/cshperspect.a031500. PMID:30104198 doi:http://dx.doi.org/10.1101/cshperspect.a031500
- ↑ Prive GG, Milburn MV, Tong L, de Vos AM, Yamaizumi Z, Nishimura S, Kim SH. X-ray crystal structures of transforming p21 ras mutants suggest a transition-state stabilization mechanism for GTP hydrolysis. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3649-53. doi: 10.1073/pnas.89.8.3649. PMID:1565661 doi:http://dx.doi.org/10.1073/pnas.89.8.3649
- ↑ Lupton CJ, Bayly-Jones C, D'Andrea L, Huang C, Schittenhelm RB, Venugopal H, Whisstock JC, Halls ML, Ellisdon AM. The cryo-EM structure of the human neurofibromin dimer reveals the molecular basis for neurofibromatosis type 1. Nat Struct Mol Biol. 2021 Dec;28(12):982-988. doi: 10.1038/s41594-021-00687-2., Epub 2021 Dec 9. PMID:34887559 doi:http://dx.doi.org/10.1038/s41594-021-00687-2
- ↑ Cimino PJ, Gutmann DH. Neurofibromatosis type 1. Handb Clin Neurol. 2018;148:799-811. doi: 10.1016/B978-0-444-64076-5.00051-X. PMID:29478615 doi:http://dx.doi.org/10.1016/B978-0-444-64076-5.00051-X
- ↑ Ly KI, Blakeley JO. The Diagnosis and Management of Neurofibromatosis Type 1. Med Clin North Am. 2019 Nov;103(6):1035-1054. doi: 10.1016/j.mcna.2019.07.004. PMID:31582003 doi:http://dx.doi.org/10.1016/j.mcna.2019.07.004
Proteopedia Page Contributors and Editors (what is this?)
Jordyn K. Lenard, Ryan D. Adkins, Michal Harel, OCA, Jaime Prilusky