User:Anna Postnikova/MAT
From Proteopedia
| Line 11: | Line 11: | ||
<StructureSection load='5a1i' size='400' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='5a1i' size='400' side='right' caption='Caption for this structure' scene=''> | ||
<scene name='90/907472/Mat2a/4'>MATa2 Subunit</scene> | <scene name='90/907472/Mat2a/4'>MATa2 Subunit</scene> | ||
| + | MAT consists of α and β subunits. MATα1 and MATα2 are catalytic subunits while MATβ is a regulatory subunit. The subunits are encoded on different genes in humans, so they are created separately and can then come together to form various complexes, such as MATαβ or MATα2 dimers <ref name="Murray et al." />. | ||
| - | <scene name='90/907472/Substrates/1'> | + | The <scene name='90/907472/Substrates/1'>substrates</scene> used by the enzyme are methionine and |
| + | and <scene name='90/907472/Product/2'>product</scene> | ||
| - | MAT consists of α and β subunits. MATα1 and MATα2 are catalytic subunits while MATβ is a regulatory subunit. The subunits are encoded on different genes in humans, so they are created separately and can then come together to form various complexes, such as MATαβ or MATα2 dimers <ref name="Murray et al." />. | ||
== Other Substrates == | == Other Substrates == | ||
Revision as of 18:50, 5 April 2022
Contents |
Methionine adenosyltransferase
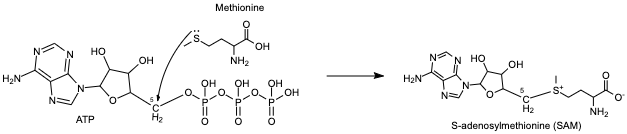
Methionine adenosyltransferase (MAT) synthesizes S-adenosylmethionine from the substrates adenosine triphosphate (ATP) and methionine. ATP isn’t used as a source of energy like it is in other reactions but gets a methionine added onto the 5th carbon while the three phosphate groups are broken down and released from the active site. This enzyme is conserved and found in many organisms, so it is essential for life. Problems with this enzyme have been shown to cause diseases such as various cancers.
SAM Formation Mechanism
Active Site Mechanism
ATP and methionine are stabilized in the active site by the residues present there, including lysine and histidine. The methionine flips toward the 5th carbon of the ATP sugar, which forms the bond to the triphosphate group. The slightly negative sulfur atom of methionine undergoes a nucleophilic attack on the slightly positive 5th carbon. Following this, the bond from the 5th carbon to the oxygen breaks, separating the three phosphates from the newly formed S-adenosylmethionine (SAM) [1].
Structure
| |||||||||||
References
- ↑ 1.0 1.1 Murray B, Antonyuk SV, Marina A, Lu SC, Mato JM, Hasnain SS, Rojas Al. Crystallography captures catalytic steps in human methionine adenosyltransferase enzymes. PNAS. 2016 Feb 8;113 (8) 2104-2109. doi: https://doi.org/10.1073/pnas.1510959113