User:Anna Postnikova/MAT
From Proteopedia
| Line 5: | Line 5: | ||
[[Image:SAM production rxn.png]] | [[Image:SAM production rxn.png]] | ||
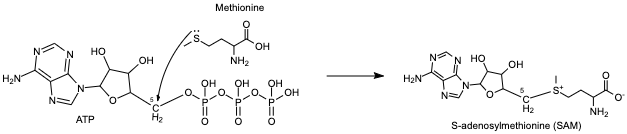
| - | + | The slightly negative sulfur atom of methionine undergoes a nucleophilic attack on the slightly positive 5th carbon of the adenosine sugar unit. Following this, the bond from the 5th carbon to the oxygen breaks, separating the tripolyphosphate from the newly formed S-adenosylmethionine (SAM) <ref name="Murray et al.">Murray B, Antonyuk SV, Marina A, Lu SC, Mato JM, Hasnain SS, Rojas Al. Crystallography captures catalytic steps in human methionine adenosyltransferase enzymes. PNAS. 2016 Feb 8;113 (8) 2104-2109. doi: https://doi.org/10.1073/pnas.1510959113</ref>. This is an example of an SN2 reaction, where an intermediate forms as the substrates are transitioning to their product forms. The product is only released after the methionine binds and the C-O bond breaks. | |
== Structure == | == Structure == | ||
<StructureSection load='5a1i' size='400' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='5a1i' size='400' side='right' caption='Caption for this structure' scene=''> | ||
<scene name='90/907472/Mat2a/4'>MATa2 Subunit</scene> | <scene name='90/907472/Mat2a/4'>MATa2 Subunit</scene> | ||
| - | MAT consists of α and β subunits. MATα1 and MATα2 are catalytic subunits while MATβ is a regulatory subunit. The subunits are encoded on different genes in humans, so they are created separately and can then come together to form various complexes, such as MATαβ or MATα2 dimers <ref name="Murray et al." />. | + | MAT consists of α and β subunits. MATα1 and MATα2 are catalytic subunits while MATβ is a regulatory subunit. Not much is currently known about the function of this regulatory subunit and how it regulates the function of the enzyme. The subunits are encoded on different genes in humans, so they are created separately and can then come together to form various complexes, such as MATαβ or MATα2 dimers <ref name="Murray et al." />. |
| - | The <scene name='90/907472/Substrates/1'>substrates</scene> used by the enzyme are methionine and | + | The <scene name='90/907472/Substrates/1'>substrates</scene> used by the enzyme are methionine and ATP. Notably, ATP is not used as a source of energy in this reaction like it is for many other processes. Instead, it is used as a substrate in the synthesis reaction. Methionine and ATP enter the active site and are stabilized by residues present there, including lysine and histidine. Once the reaction begins to take place, methionine flips toward the 5th carbon of the adenosine sugar. Following nucleophilic attack of the sulfur on the carbon, the C-O bond between the phosphates and the carbon breaks, and the <scene name='90/907472/Product/2'>products</scene> are formed. SAM is released from the active site first. The tripolyphosphate is hydrolyzed into pyrophosphate and orthophosphate and released from the active site (cite; is this catalyzed by the enzyme?) |
| - | and <scene name='90/907472/Product/2'> | + | |
Revision as of 14:59, 6 April 2022
Contents |
Methionine adenosyltransferase
Methionine adenosyltransferase (MAT) synthesizes S-adenosylmethionine from the substrates adenosine triphosphate (ATP) and methionine. ATP isn’t used as a source of energy like it is in other reactions but gets a methionine added onto the 5th carbon while the three phosphate groups are broken down and released from the active site. This enzyme is conserved and found in many organisms, so it is essential for life. Problems with this enzyme have been shown to cause diseases such as various cancers.
Active Site Mechanism
The slightly negative sulfur atom of methionine undergoes a nucleophilic attack on the slightly positive 5th carbon of the adenosine sugar unit. Following this, the bond from the 5th carbon to the oxygen breaks, separating the tripolyphosphate from the newly formed S-adenosylmethionine (SAM) [1]. This is an example of an SN2 reaction, where an intermediate forms as the substrates are transitioning to their product forms. The product is only released after the methionine binds and the C-O bond breaks.
Structure
| |||||||||||
References
- ↑ 1.0 1.1 Murray B, Antonyuk SV, Marina A, Lu SC, Mato JM, Hasnain SS, Rojas Al. Crystallography captures catalytic steps in human methionine adenosyltransferase enzymes. PNAS. 2016 Feb 8;113 (8) 2104-2109. doi: https://doi.org/10.1073/pnas.1510959113