Sandbox Reserved 1725
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
[[Image:barrel_domain_smaller.jpg|200 px|right|thumb|Figure 2. VKOR with Barrel Domain]] | [[Image:barrel_domain_smaller.jpg|200 px|right|thumb|Figure 2. VKOR with Barrel Domain]] | ||
=== Vitamin K Cycle === | === Vitamin K Cycle === | ||
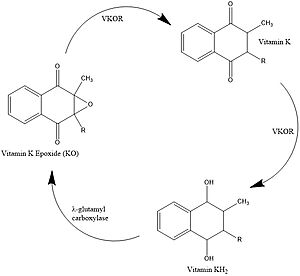
| - | [https://en.wikipedia.org/wiki/Vitamin_K Vitamin K] is essential for blood clotting in the body. The fully reduced form, KH2, | + | [https://en.wikipedia.org/wiki/Vitamin_K Vitamin K] is essential for [https://en.wikipedia.org/wiki/Coagulation#Coagulation_cascade blood clotting] in the body<ref name="Stafford">PMID:16102054</ref>. The fully reduced form, KH2, is used in the gamma carboxylation of blood clotting cofactors and is turned into the epoxide form in the process<ref name="Stafford">PMID:16102054</ref>. Vitamin K epoxide reductase, abbreviated VKOR, turns the epoxide back to the fully reduced form so the reduced form can be used again. This transformation happens in two steps: 1) converting the epoxide to the partially oxidized Vitamin K quinone and 2) converting the quinone to the fully reduced hydroquinone (KH2) (Figure 1) <ref name="Stafford">PMID:16102054</ref>. |
| - | + | ||
=== Structural Overview === | === Structural Overview === | ||
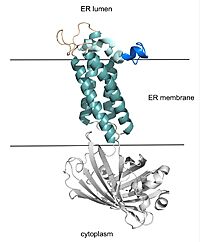
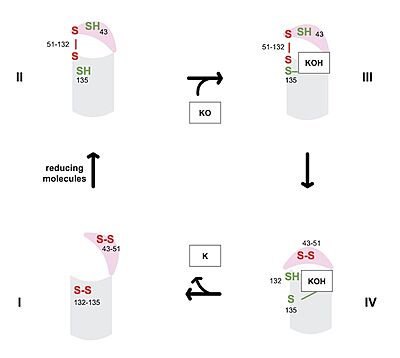
| - | VKOR consists of four <scene name='90/904330/Transmembranehelices1/1'>transmembrane helices</scene> embedded in the endoplasmic reticulum membrane. The | + | VKOR consists of four <scene name='90/904330/Transmembranehelices1/1'>transmembrane helices</scene> embedded in the endoplasmic reticulum membrane. The barrel domain was used experimentally to stabilize VKOR for structure determination (Figure 2)<ref name="Liu">PMID:33154105</ref>. For this page, the barrel domain has been removed and structures renumbered to correspond with the article by Liu. <ref name="Liu">PMID:33154105</ref>. Helices one and two are connected by the <scene name='90/904330/Betahairpin2/1'>beta hairpin</scene> region which contains two of the active cysteines, C43 and C51; these cysteines, along with C132 and C135, are essential for reduction and structural changes discussed in the next section<ref name="Liu">PMID:33154105</ref>. VKOR also has a <scene name='90/904330/Capdomain/1'>cap domain</scene> covering the active site, made up of an <scene name='90/904330/Capanchor/1'>anchor</scene>, <scene name='90/904330/Caploop/1'>loop</scene>, and <scene name='90/904330/Caphelix/1'>helix</scene>. The anchor serves to attach the cap domain to the ER membrane for stabilization<ref name="Liu">PMID:33154105</ref>. The loop helps stabilize one of the catalytic amino acids, Asn80<ref name="Liu">PMID:33154105</ref>. The helix is involved in stabilization of certain disulfide bonds and structural changes as part of the catalytic cycle discussed below<ref name="Liu">PMID:33154105</ref>. |
| + | |||
== Catalytic Cycle == | == Catalytic Cycle == | ||
Revision as of 18:47, 7 April 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
Vitamin K Epoxide Reductase
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Stafford DW. The vitamin K cycle. J Thromb Haemost. 2005 Aug;3(8):1873-8. doi: 10.1111/j.1538-7836.2005.01419.x. PMID:16102054 doi:http://dx.doi.org/10.1111/j.1538-7836.2005.01419.x
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ 3.0 3.1 Patel S, Singh R, Preuss CV, Patel N. Warfarin PMID:29261922
- ↑ Wu S, Chen X, Jin DY, Stafford DW, Pedersen LG, Tie JK. Warfarin and vitamin K epoxide reductase: a molecular accounting for observed inhibition. Blood. 2018 Aug 9;132(6):647-657. doi: 10.1182/blood-2018-01-830901. Epub 2018, May 9. PMID:29743176 doi:http://dx.doi.org/10.1182/blood-2018-01-830901
- ↑ Chong YK, Mak TW. Superwarfarin (Long-Acting Anticoagulant Rodenticides) Poisoning: from Pathophysiology to Laboratory-Guided Clinical Management. Clin Biochem Rev. 2019 Nov;40(4):175-185. doi: 10.33176/AACB-19-00029. PMID:31857739 doi:http://dx.doi.org/10.33176/AACB-19-00029
Student Contributors
Izabella Jordan, Emma Varness