We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1725
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
== Introduction == | == Introduction == | ||
Vitamin K epoxide reductase (VKOR) is the enzyme responsible for regenerating vitamin K from vitamin K epoxide to support blood coagulation. | Vitamin K epoxide reductase (VKOR) is the enzyme responsible for regenerating vitamin K from vitamin K epoxide to support blood coagulation. | ||
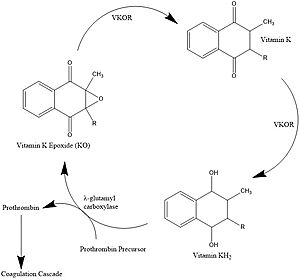
| - | [[Image: | + | [[Image:Vitamin_K_Cycle_2.jpg|300 px|left|thumb|'''Figure 1. Vitamin K Cycle''' λ-glutamyl |
carboxylase uses Vitamin KH2 to carboxylate blood clotting cofactors, converting KH2 to KO in the process. Prothrombin is shown as an example of a blood clotting cofactor that later enters the coagulation cascade. VKOR converts KO back to KH2 via two steps with Vitamin K as an intermediate.]] | carboxylase uses Vitamin KH2 to carboxylate blood clotting cofactors, converting KH2 to KO in the process. Prothrombin is shown as an example of a blood clotting cofactor that later enters the coagulation cascade. VKOR converts KO back to KH2 via two steps with Vitamin K as an intermediate.]] | ||
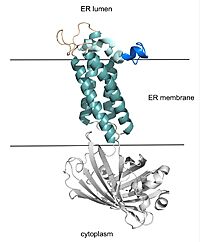
| - | [[Image:barrel_domain_smaller.jpg|200 px|right|thumb|Figure 2. VKOR with Barrel Domain]] | + | [[Image:barrel_domain_smaller.jpg|200 px|right|thumb|'''Figure 2. VKOR with Barrel Domain''' The teal helices are the transmembrane helices situated in the ER membrane. The tan sections are the beta loop and cap loop. The light blue section is the cap helix, and the dark blue section is the anchor. The cap loop, cap helix, and anchor make up the cap domain. The white barrel domain was used for stabilization during structural experiments and is not shown in future scenes on this page.]] |
=== Vitamin K Cycle === | === Vitamin K Cycle === | ||
[https://en.wikipedia.org/wiki/Vitamin_K Vitamin K] is essential for [https://en.wikipedia.org/wiki/Coagulation#Coagulation_cascade blood clotting] in the body<ref name="Stafford">PMID:16102054</ref>. The fully reduced form, KH2, is used by [https://en.wikipedia.org/wiki/Gamma-glutamyl_carboxylase#:~:text=Gamma-glutamyl%20carboxylase%20is%20an%20enzyme%20that%20catalyzes%20the,of%20the%20encoded%20enzyme%20is%20essential%20for%20hemostasis gamma-glutamyl carboxylase] to carboxylate protein-bound glutamate residues in blood clotting cofactor precursors <ref name="Blanchard">PMID:6165889</ref>. After carboxylation, the clotting cofactors (such as [https://proteopedia.org/wiki/index.php/Thrombin prothrombin]) can bind to calcium and can proceed to the coagulation cascade <ref name="Swanson">PMID:6758841</ref>. During this process, KH2 becomes oxidized to Vitamin K epoxide, or KO <ref name="Blanchard">PMID:6165889</ref>. Vitamin K epoxide reductase, abbreviated VKOR, turns the epoxide back to the fully reduced form so the reduced form can be used again. This transformation happens in two steps: 1) converting the epoxide to the partially oxidized Vitamin K quinone and 2) converting the quinone to the fully reduced hydroquinone (KH2) (Figure 1) <ref name="Stafford">PMID:16102054</ref>. | [https://en.wikipedia.org/wiki/Vitamin_K Vitamin K] is essential for [https://en.wikipedia.org/wiki/Coagulation#Coagulation_cascade blood clotting] in the body<ref name="Stafford">PMID:16102054</ref>. The fully reduced form, KH2, is used by [https://en.wikipedia.org/wiki/Gamma-glutamyl_carboxylase#:~:text=Gamma-glutamyl%20carboxylase%20is%20an%20enzyme%20that%20catalyzes%20the,of%20the%20encoded%20enzyme%20is%20essential%20for%20hemostasis gamma-glutamyl carboxylase] to carboxylate protein-bound glutamate residues in blood clotting cofactor precursors <ref name="Blanchard">PMID:6165889</ref>. After carboxylation, the clotting cofactors (such as [https://proteopedia.org/wiki/index.php/Thrombin prothrombin]) can bind to calcium and can proceed to the coagulation cascade <ref name="Swanson">PMID:6758841</ref>. During this process, KH2 becomes oxidized to Vitamin K epoxide, or KO <ref name="Blanchard">PMID:6165889</ref>. Vitamin K epoxide reductase, abbreviated VKOR, turns the epoxide back to the fully reduced form so the reduced form can be used again. This transformation happens in two steps: 1) converting the epoxide to the partially oxidized Vitamin K quinone and 2) converting the quinone to the fully reduced hydroquinone (KH2) (Figure 1) <ref name="Stafford">PMID:16102054</ref>. | ||
Revision as of 19:51, 7 April 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
Vitamin K Epoxide Reductase
| |||||||||||
References
- ↑ 1.0 1.1 Stafford DW. The vitamin K cycle. J Thromb Haemost. 2005 Aug;3(8):1873-8. doi: 10.1111/j.1538-7836.2005.01419.x. PMID:16102054 doi:http://dx.doi.org/10.1111/j.1538-7836.2005.01419.x
- ↑ 2.0 2.1 Blanchard RA, Furie BC, Jorgensen M, Kruger SF, Furie B. Acquired vitamin K-dependent carboxylation deficiency in liver disease. N Engl J Med. 1981 Jul 30;305(5):242-8. doi: 10.1056/NEJM198107303050502. PMID:6165889 doi:http://dx.doi.org/10.1056/NEJM198107303050502
- ↑ Swanson JC, Suttie JW. Vitamin K dependent in vitro production of prothrombin. Biochemistry. 1982 Nov 9;21(23):6011-8. doi: 10.1021/bi00266a044. PMID:6758841 doi:http://dx.doi.org/10.1021/bi00266a044
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ 5.0 5.1 Patel S, Singh R, Preuss CV, Patel N. Warfarin PMID:29261922
- ↑ Wu S, Chen X, Jin DY, Stafford DW, Pedersen LG, Tie JK. Warfarin and vitamin K epoxide reductase: a molecular accounting for observed inhibition. Blood. 2018 Aug 9;132(6):647-657. doi: 10.1182/blood-2018-01-830901. Epub 2018, May 9. PMID:29743176 doi:http://dx.doi.org/10.1182/blood-2018-01-830901
- ↑ Chong YK, Mak TW. Superwarfarin (Long-Acting Anticoagulant Rodenticides) Poisoning: from Pathophysiology to Laboratory-Guided Clinical Management. Clin Biochem Rev. 2019 Nov;40(4):175-185. doi: 10.33176/AACB-19-00029. PMID:31857739 doi:http://dx.doi.org/10.33176/AACB-19-00029
Student Contributors
Izabella Jordan, Emma Varness