We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Neurofibromin
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
====Conformations==== | ====Conformations==== | ||
Neurofibromin is a dimeric protein that is found in the cytoplasm. It exists in two conformations, <scene name='90/904326/Open_conformation/2'>open</scene> and <scene name='90/904326/Closed_conformation/2'>closed</scene> . The open conformation has one of the protomers in an auto-inhibited conformation and the other in an open conformation. In the <scene name='90/904326/Open_conformation_with_grd_hig/2'>open conformation</scene>, Ras is able to bind to the GRD neurofibromin. The <scene name='90/904326/Grd_closed_conformation/2'>closed conformation</scene> has both protomers in an autoinhibited conformation, which sterically hinders the binding of Ras to GRD. Only one of the protomers has to be in the open conformation for Ras to bind. | Neurofibromin is a dimeric protein that is found in the cytoplasm. It exists in two conformations, <scene name='90/904326/Open_conformation/2'>open</scene> and <scene name='90/904326/Closed_conformation/2'>closed</scene> . The open conformation has one of the protomers in an auto-inhibited conformation and the other in an open conformation. In the <scene name='90/904326/Open_conformation_with_grd_hig/2'>open conformation</scene>, Ras is able to bind to the GRD neurofibromin. The <scene name='90/904326/Grd_closed_conformation/2'>closed conformation</scene> has both protomers in an autoinhibited conformation, which sterically hinders the binding of Ras to GRD. Only one of the protomers has to be in the open conformation for Ras to bind. | ||
| + | ====SPRED-1 Protein==== | ||
| + | The SPRED-1 protein localizes the Neurofibromin protein to the cell membrane in to allow it to bind to the membrane oriented Ras protein<ref name= ''Naschberger''>PMID:34707296</ref>. | ||
====Ras binding site==== | ====Ras binding site==== | ||
| Line 23: | Line 25: | ||
== Disease Relevance == | == Disease Relevance == | ||
| - | Mutations to the neurofibromin protein are implicated in the progression of Neurofibromatosis type 1. Neurofibromatosis type 1 is a condition where cancer develops by inactivating the Ras suppression effects of NF, allowing Ras to behave as an oncogene. Neurofibromatosis type 1 is an autosomal dominant disorder that affects 1 in 3,000 people. The ''NF'' gene has the highest mutation rate of any human gene, adding to the prevalence of cancers related to neurofibromin mutations<ref name= ''Lupton''>PMID:34887559</ref>. Furthermore, these mutations consist heavily of [https://en.wikipedia.org/wiki/Mutation#By_inheritance ''de novo''] mutations<ref name= ''Abramowicz''>PMID:25182393</ref>. NF1 primarily causes tumors in the [https://en.wikipedia.org/wiki/Central_nervous_system_disease#Structural_defects central] and peripheral nervous systems, but often has a multisystem expression including tumors in the dermatologic, cardiovascular, gastrointestinal, and orthopedic systems<ref name= ''Cimino''>PMID:29478615</ref>. The wide range of presentations is consistent with the multiplicity of mutations observed in the causative protein<ref name= ''Ly''>PMID:31582003</ref>. This multiplicity derives from the immense size and homo dimeric nature of the neurofibromin protein that allows for otherwise innocuous mutations to wreak havoc on the conformations of the protein as well as its ability to bind to the Ras protein given that the positioning of NF | + | Mutations to the neurofibromin protein are implicated in the progression of Neurofibromatosis type 1. Neurofibromatosis type 1 is a condition where cancer develops by inactivating the Ras suppression effects of NF, allowing Ras to behave as an oncogene. Neurofibromatosis type 1 is an autosomal dominant disorder that affects 1 in 3,000 people. The ''NF'' gene has the highest mutation rate of any human gene, adding to the prevalence of cancers related to neurofibromin mutations<ref name= ''Lupton''>PMID:34887559</ref>. Furthermore, these mutations consist heavily of [https://en.wikipedia.org/wiki/Mutation#By_inheritance ''de novo''] mutations<ref name= ''Abramowicz''>PMID:25182393</ref>. NF1 primarily causes tumors in the [https://en.wikipedia.org/wiki/Central_nervous_system_disease#Structural_defects central] and peripheral nervous systems, but often has a multisystem expression including tumors in the dermatologic, cardiovascular, gastrointestinal, and orthopedic systems<ref name= ''Cimino''>PMID:29478615</ref>. The wide range of presentations is consistent with the multiplicity of mutations observed in the causative protein<ref name= ''Ly''>PMID:31582003</ref>. This multiplicity derives from the immense size and homo dimeric nature of the neurofibromin protein that allows for otherwise innocuous mutations to wreak havoc on the conformations of the protein as well as its ability to bind to the Ras protein given that the positioning of NF relative to Ras is highly important. |
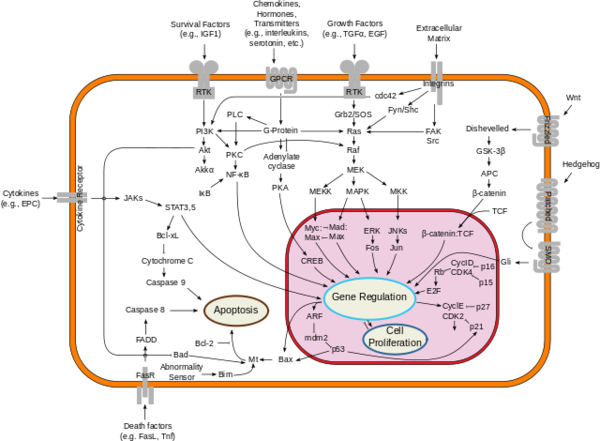
==Downstream Effects== | ==Downstream Effects== | ||
Revision as of 20:04, 7 April 2022
| |||||||||||
References
- ↑ Bergoug M, Doudeau M, Godin F, Mosrin C, Vallee B, Benedetti H. Neurofibromin Structure, Functions and Regulation. Cells. 2020 Oct 27;9(11). pii: cells9112365. doi: 10.3390/cells9112365. PMID:33121128 doi:http://dx.doi.org/10.3390/cells9112365
- ↑ Naschberger A, Baradaran R, Rupp B, Carroni M. The structure of neurofibromin isoform 2 reveals different functional states. Nature. 2021 Nov;599(7884):315-319. doi: 10.1038/s41586-021-04024-x. Epub 2021, Oct 27. PMID:34707296 doi:http://dx.doi.org/10.1038/s41586-021-04024-x
- ↑ Trovo-Marqui AB, Tajara EH. Neurofibromin: a general outlook. Clin Genet. 2006 Jul;70(1):1-13. doi: 10.1111/j.1399-0004.2006.00639.x. PMID:16813595 doi:http://dx.doi.org/10.1111/j.1399-0004.2006.00639.x
- ↑ Hall BE, Bar-Sagi D, Nassar N. The structural basis for the transition from Ras-GTP to Ras-GDP. Proc Natl Acad Sci U S A. 2002 Sep 17;99(19):12138-42. Epub 2002 Sep 4. PMID:12213964 doi:http://dx.doi.org/10.1073/pnas.192453199
- ↑ Cimino PJ, Gutmann DH. Neurofibromatosis type 1. Handb Clin Neurol. 2018;148:799-811. doi: 10.1016/B978-0-444-64076-5.00051-X. PMID:29478615 doi:http://dx.doi.org/10.1016/B978-0-444-64076-5.00051-X
- ↑ Naschberger A, Baradaran R, Rupp B, Carroni M. The structure of neurofibromin isoform 2 reveals different functional states. Nature. 2021 Nov;599(7884):315-319. doi: 10.1038/s41586-021-04024-x. Epub 2021, Oct 27. PMID:34707296 doi:http://dx.doi.org/10.1038/s41586-021-04024-x
- ↑ Frech M, Darden TA, Pedersen LG, Foley CK, Charifson PS, Anderson MW, Wittinghofer A. Role of glutamine-61 in the hydrolysis of GTP by p21H-ras: an experimental and theoretical study. Biochemistry. 1994 Mar 22;33(11):3237-44. doi: 10.1021/bi00177a014. PMID:8136358 doi:http://dx.doi.org/10.1021/bi00177a014
- ↑ Bunda S, Burrell K, Heir P, Zeng L, Alamsahebpour A, Kano Y, Raught B, Zhang ZY, Zadeh G, Ohh M. Inhibition of SHP2-mediated dephosphorylation of Ras suppresses oncogenesis. Nat Commun. 2015 Nov 30;6:8859. doi: 10.1038/ncomms9859. PMID:26617336 doi:http://dx.doi.org/10.1038/ncomms9859
- ↑ Lupton CJ, Bayly-Jones C, D'Andrea L, Huang C, Schittenhelm RB, Venugopal H, Whisstock JC, Halls ML, Ellisdon AM. The cryo-EM structure of the human neurofibromin dimer reveals the molecular basis for neurofibromatosis type 1. Nat Struct Mol Biol. 2021 Dec;28(12):982-988. doi: 10.1038/s41594-021-00687-2., Epub 2021 Dec 9. PMID:34887559 doi:http://dx.doi.org/10.1038/s41594-021-00687-2
- ↑ Abramowicz A, Gos M. Neurofibromin in neurofibromatosis type 1 - mutations in NF1gene as a cause of disease. Dev Period Med. 2014 Jul-Sep;18(3):297-306. PMID:25182393
- ↑ Cimino PJ, Gutmann DH. Neurofibromatosis type 1. Handb Clin Neurol. 2018;148:799-811. doi: 10.1016/B978-0-444-64076-5.00051-X. PMID:29478615 doi:http://dx.doi.org/10.1016/B978-0-444-64076-5.00051-X
- ↑ Ly KI, Blakeley JO. The Diagnosis and Management of Neurofibromatosis Type 1. Med Clin North Am. 2019 Nov;103(6):1035-1054. doi: 10.1016/j.mcna.2019.07.004. PMID:31582003 doi:http://dx.doi.org/10.1016/j.mcna.2019.07.004
- ↑ McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007 Aug;1773(8):1263-84. doi:, 10.1016/j.bbamcr.2006.10.001. Epub 2006 Oct 7. PMID:17126425 doi:http://dx.doi.org/10.1016/j.bbamcr.2006.10.001
Proteopedia Page Contributors and Editors (what is this?)
Jordyn K. Lenard, Ryan D. Adkins, Michal Harel, OCA, Jaime Prilusky