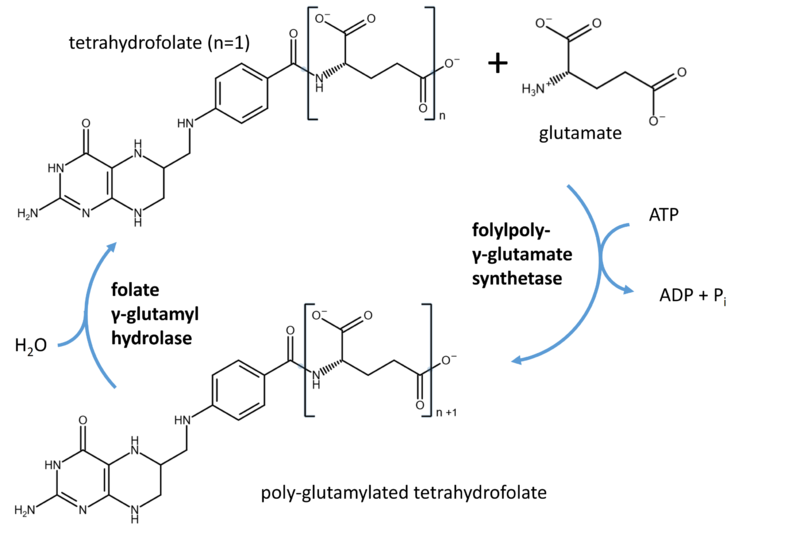
Folylpolyglutamate synthetase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| + | [[Folylpolyglutamate synthetase]] (FPGS) attaches gamma-glutamate units to various forms of folate (vitamin B9). In eukaryotic organisms, polyglutamylation keeps folate in the compartment, while folate lacking it can travel to other compartments. To be effecitive, antifolates (competitive inhibitors whose structure is similar to tetrahydrofolate) have to undergo polyglutamylation as well. Thus, defects in FPGS have consequences for the one-carbon metabolism relying on availability of folate as well as treatment of cancer relying on antifolates. | ||
| + | |||
| + | ### Reaction | ||
[[Image:Tetrahydrofolate synthase hydrolase.PNG|800px]] | [[Image:Tetrahydrofolate synthase hydrolase.PNG|800px]] | ||
| + | |||
| + | ### FPGS in different organisms | ||
EC numbers: 6.3.2.12 (DHF synthase) and 6.3.2.17 (poly) | EC numbers: 6.3.2.12 (DHF synthase) and 6.3.2.17 (poly) | ||
| Line 19: | Line 24: | ||
Primary citation of [[1w78]] <ref>PMID:15705579</ref> | Primary citation of [[1w78]] <ref>PMID:15705579</ref> | ||
| - | == References == | ||
| - | |||
| - | <references/> | ||
==Your Heading Here (maybe something like 'Structure')== | ==Your Heading Here (maybe something like 'Structure')== | ||
<StructureSection load='1W78' size='340' side='right' caption='FolC' scene=''> | <StructureSection load='1W78' size='340' side='right' caption='FolC' scene=''> | ||
| - | This is a default text for your page '''Karsten Theis/FolC'''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | ||
| - | You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | ||
| - | |||
| - | == Function == | ||
| - | |||
| - | == Disease == | ||
| - | |||
| - | == Relevance == | ||
| - | |||
| - | == Structural highlights == | ||
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 02:11, 11 April 2022
Folylpolyglutamate synthetase (FPGS) attaches gamma-glutamate units to various forms of folate (vitamin B9). In eukaryotic organisms, polyglutamylation keeps folate in the compartment, while folate lacking it can travel to other compartments. To be effecitive, antifolates (competitive inhibitors whose structure is similar to tetrahydrofolate) have to undergo polyglutamylation as well. Thus, defects in FPGS have consequences for the one-carbon metabolism relying on availability of folate as well as treatment of cancer relying on antifolates.
- Reaction
- FPGS in different organisms
EC numbers: 6.3.2.12 (DHF synthase) and 6.3.2.17 (poly)
Precursor:
Reaction:
Compartments[1]
Rheumatoid arthritis[2]
Substrate binding and mechanism (1jbv and 1jbw): [3]
Your Heading Here (maybe something like 'Structure')
| |||||||||||
References
- ↑ Jackman AL, Calvert AH. Folate-based thymidylate synthase inhibitors as anticancer drugs. Ann Oncol. 1995 Nov;6(9):871-81. doi: 10.1093/oxfordjournals.annonc.a059353. PMID:8624289 doi:http://dx.doi.org/10.1093/oxfordjournals.annonc.a059353
- ↑ Muller IB, Lin M, Lems WF, Ter Wee MM, Wojtuszkiewicz A, Nurmohamed MT, Cloos J, Assaraf YG, Jansen G, de Jonge R. Association of altered folylpolyglutamate synthetase pre-mRNA splicing with methotrexate unresponsiveness in early rheumatoid arthritis. Rheumatology (Oxford). 2021 Mar 2;60(3):1273-1281. doi:, 10.1093/rheumatology/keaa428. PMID:32940699 doi:http://dx.doi.org/10.1093/rheumatology/keaa428
- ↑ Sun X, Cross JA, Bognar AL, Baker EN, Smith CA. Folate-binding triggers the activation of folylpolyglutamate synthetase. J Mol Biol. 2001 Jul 27;310(5):1067-78. PMID:11501996 doi:10.1006/jmbi.2001.4815
- ↑ Mathieu M, Debousker G, Vincent S, Viviani F, Bamas-Jacques N, Mikol V. Escherichia coli FolC structure reveals an unexpected dihydrofolate binding site providing an attractive target for anti-microbial therapy. J Biol Chem. 2005 May 13;280(19):18916-22. Epub 2005 Feb 10. PMID:15705579 doi:10.1074/jbc.M413799200