This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1701
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | {{Template:CH462_Biochemistry_II_2022}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
= MRGPRX2 Human Itch G-Protein Coupled Receptor (GPCR) = | = MRGPRX2 Human Itch G-Protein Coupled Receptor (GPCR) = | ||
| - | <StructureSection load='7s8l' size=' | + | <StructureSection load='7s8l' size='350' frame='true' |
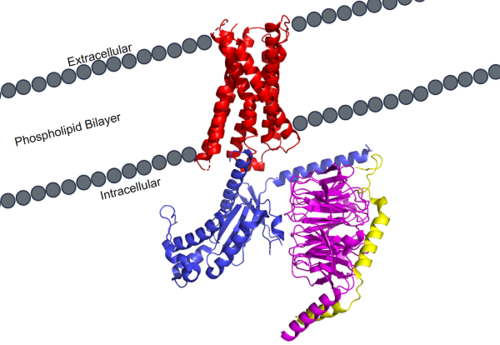
| - | + | side='right' caption='MRGPRX2 is a specific type of GPCR. Its transmembrane domain (red) spans the phospholipid bilayer of cellular membranes and attaches to the G-protein. The G-protein consists of 3 different domains: alpha (blue), beta (magenta), and gamma (yellow). scene ='90/904305/Structure_overview/2'> | |
| - | + | MRGPRX2 is a certain type of [https://proteopedia.org/wiki/index.php/G_protein-coupled_receptor GPCR] that is located in the cellular membranes of mast cells. | |
| - | MRGPRX2 | + | [[Image:MRGPRX2_within_membrane.png|500px|right|thumb|'''Figure 1.''' MRGPRX2 as it sits within the cellular membrane. Phospholipid bilayer is represented by grey dots, with labeled cellular locations]] |
| - | [[Image: | + | |
| + | == Background == | ||
| + | GCPR’s or G-Protein Coupled Receptors are a special type of protein receptor that promotes cellular signaling. Due to its structure spanning the cellular membrane, it works to transmit extracellular signals to create a change inside the cell. This is called signal transduction, and is a common way extracellular signals produce a change within cells. Some common cellular pathways that utilize GPCR’s are found in [https://proteopedia.org/wiki/index.php/Rhodopsin Rhodopsin], a protein essential for the human vision response, or the [https://proteopedia.org/wiki/index.php/Beta2_adrenergic_receptor-Gs_protein_complex_updated adrenaline fight-or-flight response]. Understanding GPCR’s and how they produce their desired intracellular signal is essential understanding essential cellular pathways, especially in their diseased states. As of 2017, there were about 475 drugs in circulation that acted on 108 GPCR targets, and at least 321 GPCR-targeting drugs were in clinical trial stages, with about 20% of them targeting novel GPCR’s. <ref name="Hauser">PMID:29075003</ref> Because of the clinical relevance of GPCR’s, every new structure found, such as MRGPRX2, contributes to essential drug development to both treat disease, or modulate harmful side effects. | ||
| + | Because of how many different types of GPCR’s there are, they have been categorized into 6 different classes based on shared sequences and functions.<ref name="Basith">PMID:29593527</ref> MRGPRX2 is categorized into the [https://proteopedia.org/wiki/index.php/GPCR#Family_A_of_GPCRs Class A] receptor family, however it has important differences that make it a unique type of Class A receptor. <ref name="Cao">PMID: 34789874</ref><ref name="Yang">PMID: 34789875</ref> | ||
| + | == GPCR Structure == | ||
| + | Cryo-electron microscopy (cryo-EM) was used to image the MRGPRX2 receptor to analyze its structure. <ref name= "Cao" /> <ref name= "Yang" /> which helped to classify it into the A family of GPCR's. MRGPRX2 therefore shares the same general structural domains of all GPCR's. This includes a <scene name='90/904305/Structure_overview_red/1'>transmembrane domain</scene> and a <scene name='90/904305/Structure_overview_gprotein/1'>G-protein</scene> domain. The G-protein domain consists of <scene name='90/904305/Structure_overview_alpha/1'>alpha</scene>, <scene name='90/904305/Structure_overview_beta/1'>beta</scene>, and <scene name='90/904305/Structure_overview_gamma/1'>gamma</scene> subunits. In preparing the protein sample, MRGPRX2 was prepared with the <scene name='90/904305/Antibody_representation/1'>antibody scFv16</scene> in order to stabilize the membrane for proper imaging. For simplicity and to focus on the specific MRGPRX2 receptor, the antibody has been removed. | ||
| - | + | === Transmembrane Domain === | |
| + | The transmembrane domain spans the cellular membrane. It consists of <scene name='90/904305/Transmembrane_protein_c_and_l/1'>seven transmembrane helices</scene> and <scene name='90/904305/Ecl_and_icl/2'>6 loops</scene> (three extracellular loops, and three intracellular loops). The transmembrane helices are numbered 1-7 and contain special conserved motifs that are shared across other A family receptors. These motifs are expanded upon later, as they heavily contribute to the structure and therefore function of the transmembrane domain as a whole. | ||
| + | The extracellular domain of the protein is responsible for ligand binding, which initiates signal transduction. The properties of the extracellular environment of the transmembrane domain determines what ligands bind to the protein, and what type of binding interactions the protein and ligand have. In MRGPRX2, the extracellular side of the protein has one binding pocket with <scene name='90/904305/Subpockets_1_and_2/2'>two sub-pockets</scene>. Sub-pocket 1 is negatively charged due to negatively charged <scene name='90/904305/Subpockets_1_and_2_d_and_e/1'>Aspartate and Glutamate</scene> residues (D184 and E164), while sub-pocket 2 contains hydrophobic amino acids which contribute to hydrophobic interactions between the ligand and protein. | ||
| + | The intracellular domain is what connects the transmembrane and G-protein domains together. There are a wide variety of residues and important interactions that contribute to this interaction, and it is very important in transmitting the extracellular signal of ligand binding to the intracellular environment where the G-protein binds and can become activated. | ||
| - | + | === G-Protein === | |
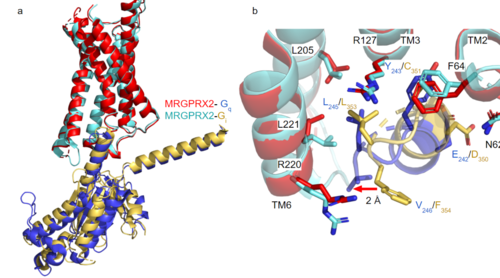
| - | + | [https://proteopedia.org/wiki/index.php/G_protein GTP-binding proteins], also known as G-proteins, are heterotrimeric complexes consisting of alpha, beta, and gamma subunits that interact with membrane receptor proteins. G-proteins are responsible transmitting extracellular signals into the cell upon activation. This activation happens when the alpha subunit of the G-protein binds GTP instead of GDP, and then disassociates from the rest of the protein, initiating the intracellular signaling cascade. There are different families of G-alpha subunits, Gαi, Gαs, Gα12/13, and Gαq <ref name="Kamato">PMID: 26664886</ref>. MRGPRX2 has been found to bind both Gαi and Gαq subunits with relatively no major structural changes between the two despite slightly different amino acids present <ref name= "Cao" /> <ref name= "Yang" />. Throughout this page, MGPRX2 is always shown with Gq. '''Figure 2a''' shows the overlay of MGPRX2 with either the Gq or Gi alpha subunit. '''Figure 2b''' shows the specific residues involved in the <scene name='90/904306/Interface_2/1'>interface</scene> between the membrane receptor and G protein. The major difference between the two comes from one amino acid difference (valine on Gq versus phenylalanine on Gi) that pushes the Gi subunit 2Å away from the arginine residue on helix 6 of the transmembrane protein. This is the only major structural difference between Gq and Gi subunits. | |
| - | + | ||
| + | [[Image:Gq and gi overlay.png|500px|center|thumb|'''Figure 2a.''' Overlay of MGPRX2-Gq (red-dark blue) and MGPRX2-Gi (cyan-yellow). '''Figure 2b.''' Important residues involved in the interface between MGPRX2 and Gq/ Gi subunits. Arrow pointing to the major difference between the interfaces, which comes from the final C-terminus residue on the G-alpha subunit. In Gq, there is a valine while in Gi, there is a phenylalanine. This pushes the Gi subunit 2Å away from the arginine residue on helix 6 of the transmembrane protein.]] | ||
| - | == Structure == | ||
| - | === Heterotrimeric G-Protein Structure === | ||
| - | <scene name='90/904306/Interface/1'>Text To Be Displayed</scene> | ||
| - | <scene name='90/904306/Interface_2/1'>interface</scene> | ||
| - | <scene name='90/904306/Interface_2/1'>interface</scene> | ||
=== Novel Characteristics === | === Novel Characteristics === | ||
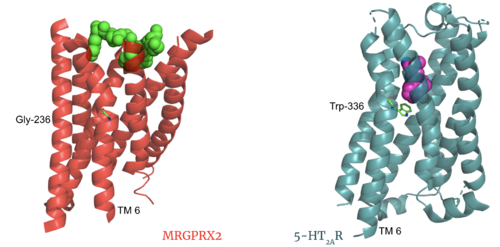
| - | MRGPRX2 | + | The most important findings about the MRGPRX2 receptor are the differences between it and all other previously discovered GPCR's found in the A family. Many conserved structural motifs, characteristic of the A family receptors, are absent on MRGPRX2. These structural motif differences contribute to a membrane surface ligand binding rather than a ligand binding deep within the helices ('''Figure 3'''). To demonstrate this difference in depth binding, MRGPRX2 is compared to [https://proteopedia.org/wiki/index.php/Serotonin_receptor 5-HT2AR], another class A GPCR with more conserved structural motifs. |
---- | ---- | ||
---- | ---- | ||
| - | [[Image:Screen Shot 2022-03-27 at 5.06.17 PM.png|500px|center|thumb|Comparison of ligand Cortistatin-14 binding in MRGPRX2 (left) and binding in 5HT2AR (right)]] | + | [[Image:Screen Shot 2022-03-27 at 5.06.17 PM.png|500px|center|thumb|'''Figure 3.''' Comparison of ligand Cortistatin-14 binding in MRGPRX2 (left) and binding in 5HT2AR (right)]] |
| Line 35: | Line 37: | ||
---- | ---- | ||
---- | ---- | ||
| + | |||
<jmol> | <jmol> | ||
<jmolButton> | <jmolButton> | ||
| Line 40: | Line 43: | ||
</jmolButton> | </jmolButton> | ||
</jmol> | </jmol> | ||
| + | |||
| + | |||
==== Toggle Switch ==== | ==== Toggle Switch ==== | ||
| + | Certain residues that lie in the ligand binding pocket can act as molecular switches to turn the GPCR “on” or “off” and are fittingly called toggle switches. Toggle switches in receptors are essential in interacting with the ligand upon binding, as they can then initiate the transmission of the molecular signal through the protein. Trp-336 has been known to act as this "iconic" toggle switch in GPCR’s of the A family <ref name="Trzaskowski">PMID: 22300046</ref>, and is also an important residue in another motif, known as the '''CWxP motif'''. However in MRGPRX2, the residue has been replaced to a <scene name='90/904305/Glycine_toggle_switch/7'>glycine</scene> <ref name="Cao">PMID: 34789874</ref> <ref name="Yang">PMID: 34789875</ref>. This is a significant modification to the receptor structure. By replacing the large tryptophan residue with a small glycine, the membrane helicies, especially helix 7 on which the toggle switch is found, can pack closer together. The ligands that interact with MRGPRX2 then bind much <scene name='90/904305/Glycine_toggle_switch_and_cor/1'>closer to the surface</scene> of the receptor, as opposed to deeper within the helices. This significant change can be visualized in '''Figure 3'''. This significantly changes what structures of ligands are able to interact with this receptor and therefore what types of molecules can activate the Human Itch GPCR. More details about what kinds of ligands bind to this receptor are discussed later. | ||
| + | |||
| + | ==== Sodium Site ==== | ||
| + | The MRGPRX2 <scene name='90/904306/Sodium_site_2/1'>sodium binding site</scene> consists of ASP-75 and GLY-116 compared to the [https://proteopedia.org/wiki/index.php/Neurotensin_receptor#sodium%20binding%20pocket previously conserved] residues in this binding pocket. Other class A GPCRs demonstrate a larger binding pocket with a higher negative character allowing for a suitable environment for sodium ions to bind. In MRGPRX2, this pocket lacks the same amount of <scene name='90/904306/Sodium_site_charge/2'>negative character</scene> with the shift to a glycine residue rather than typical negative residues. The helices for the MRGPRX2 in the binding pocket are also more collapsed making this pocket less accessible for sodium ions. | ||
==== PIF/LLF Motif ==== | ==== PIF/LLF Motif ==== | ||
| + | <jmol> | ||
| + | <jmolButton> | ||
| + | <script>moveto 1.0 { -300 -230 926 146.07} 197.11 29.14 -10.71 {120.12435037299738 118.31838742815191 99.66549773755663} 68.32091981812115 {0 0 0} 0 0 0 3.0 0.0 0.0 </script> <text>🔎Reset Orientation for Transmembrane Domain Extracellular View</text> | ||
| + | </jmolButton> | ||
| + | </jmol> | ||
| + | |||
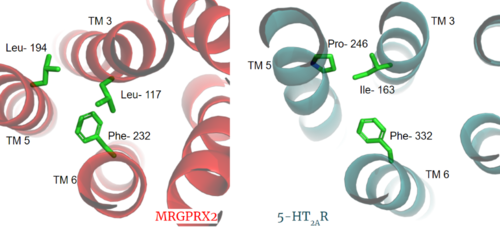
| + | Another motif found in most, but not all, A family GPCR’s is the PIF motif. The three residues are found on transmembrane helices 5, 3, and 6, respectively. In MRGPRX2, the PIF motif is changed to LLF residues. '''Figure 3''' shows the conserved PIF motif on 5HT2AR, compared to the LLF motif on MRGPRX2, found at <scene name='90/904305/Llf_motif/3'>Leu-117, Leu-194, and Phe-232</scene> on transmembrane helices 5, 3, and 6, respectively. This contributes to shifting helix 6 towards helix 3, and contributes to the tighter packing of helices <ref name="Cao">PMID: 34789874</ref> <ref name="Yang">PMID: 34789875</ref> and therefore a more surface-level ligand binding site. | ||
| + | |||
| + | [[Image:PIF_resized.png|500px|center|thumb|'''Figure 3.''' Conserved PIF motif in 5HT2AR (teal) compared to the LLF motif found in MRGPRX2 (red). Transmembrane helices and residues are numbered and labeled to show how this structural change shifts the orientation of the helices.]] | ||
| + | |||
==== DRY/ ERC Motif ==== | ==== DRY/ ERC Motif ==== | ||
MRGPRX2 has an <scene name='90/904306/Erc_motif_3/1'>ERC Motif</scene> rather than the typically [https://proteopedia.org/wiki/index.php/A_Physical_Model_of_the_%CE%B22-Adrenergic_Receptor#conserved%20DRY%20motif conserved E/DRY Motif]. The amino acid residue shift from TYR-174 to CYS-128 has spatial arrangement implications where the helices are more compact in MRGPRX2 without the TYR to physically push the TMP helices apart. | MRGPRX2 has an <scene name='90/904306/Erc_motif_3/1'>ERC Motif</scene> rather than the typically [https://proteopedia.org/wiki/index.php/A_Physical_Model_of_the_%CE%B22-Adrenergic_Receptor#conserved%20DRY%20motif conserved E/DRY Motif]. The amino acid residue shift from TYR-174 to CYS-128 has spatial arrangement implications where the helices are more compact in MRGPRX2 without the TYR to physically push the TMP helices apart. | ||
| - | [[Image:Screen Shot 2022-03-15 at 10.23.20 AM.png|200px|left|thumb|ERC Motif]] | + | [[Image:Screen Shot 2022-03-15 at 10.23.20 AM.png|200px|left|thumb|'''Figure 4.''' ERC Motif]] |
| Line 66: | Line 85: | ||
| - | ==== Sodium Site ==== | ||
| - | The MRGPRX2 <scene name='90/904306/Sodium_site_2/1'>sodium binding site</scene> consists of ASP-75 and GLY-116 compared to the [https://proteopedia.org/wiki/index.php/Neurotensin_receptor#sodium%20binding%20pocket previously conserved] residues in this binding pocket. Other class A GPCRs demonstrate a larger binding pocket with a higher negative character allowing for a suitable environment for sodium ions to bind. In MRGPRX2, this pocket lacks the same amount of <scene name='90/904306/Sodium_site_charge/2'>negative character</scene> with the shift to a glycine residue rather than typical negative residues. The helices for the MRGPRX2 in the binding pocket are also more collapsed making this pocket less accessible for sodium ions. | ||
| - | ==== Disulfide Bonds ==== | ||
| - | The MRGPRX2 disulfide bond is between CYS-168 and CYS-180 on TM helices 5 and 4, respectively. In other class A GPCRs, this disulfide bond is between and extracellular loop (ECL2) and a TM helix (TM3). For example, the <scene name='90/904306/5ht2a_disulfide/2'>serotonin GPCR</scene> shows this disulfide bond between the ECL2 and TM3. This different disulfide bond location contributes to surface level binding of ligands. | ||
| - | [[Image:Screen Shot 2022-03-27 at 5.45.52 PM.png|300px|center|thumb|Overlay of the 5HT2AR and MRGPRX2 TMP for comparison of disulfide bond location.]] | ||
| + | |||
| + | |||
| + | ==== Disulfide Bonds ==== | ||
| + | The MRGPRX2 disulfide bond is between <scene name='90/904305/Disulfide_bond/1'>Cys-168 and Cys-180</scene> on TM helices 5 and 4, respectively. In other class A GPCRs, this disulfide bond is between and extracellular loop (ECL2) and a TM helix (TM3). For example, the <scene name='90/904306/5ht2a_disulfide/2'>serotonin GPCR</scene> shows this disulfide bond between the ECL2 and TM3. This different disulfide bond location contributes to surface level binding of ligands. | ||
| + | [[Image:Screen Shot 2022-03-27 at 5.45.52 PM.png|300px|center|thumb|'''Figure 5.''' Overlay of the 5HT2AR and MRGPRX2 TMP for comparison of disulfide bond location.]] | ||
| Line 84: | Line 103: | ||
=====CWxP Motif===== | =====CWxP Motif===== | ||
The <scene name='90/904306/Cpxw_motif_2/1'>CWxP Motif</scene> is almost fully conserved except for TRP-236, or the toggle switch, which is replaced with GLY-236. CYS-235, LEU-237, and PRO-238 are all conserved. CYS6.47 may play a fundamental role in GPCR activity which would be supported by its conservation in MRGPRX2<ref name="Olivella>PMID:23497259</ref>. | The <scene name='90/904306/Cpxw_motif_2/1'>CWxP Motif</scene> is almost fully conserved except for TRP-236, or the toggle switch, which is replaced with GLY-236. CYS-235, LEU-237, and PRO-238 are all conserved. CYS6.47 may play a fundamental role in GPCR activity which would be supported by its conservation in MRGPRX2<ref name="Olivella>PMID:23497259</ref>. | ||
| + | |||
| + | === Ligands === | ||
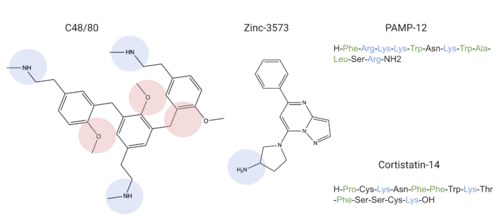
| + | MRGPRX2 binds a wide range of small molecule and peptide ligands. These ligands have positively charged regions which can interact with the negative binding pocket, sub-pocket 1. Some of these larger ligands also have a hydrophobic region which can interact with sub-pocket 2. MRGPRX2 has been known to interact with [https://pubchem.ncbi.nlm.nih.gov/compound/PAMP-12-_human_-porcine#section=Structures PAMP-12], [https://pubchem.ncbi.nlm.nih.gov/compound/44208884 Cortistatin-14], [https://pubchem.ncbi.nlm.nih.gov/compound/118797323 C48/80], and [https://pubchem.ncbi.nlm.nih.gov/compound/95882507 Zinc-3573]<ref name="Yang">PMID: 34789875</ref>,<ref name="Cao">PMID: 34789874</ref>. | ||
| + | [[Image:Screen Shot 2022-03-29 at 9.15.16 AM.png PM.png|500px|center|thumb|Common MRGPRX2 ligands with positive regions in blue, negative regions in red, and hydrophobic regions in green]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | Ligands' charge or hydrophilicity are shown to demonstrate where they may interact with sub-pockets 1 and 2. <scene name='90/904306/C-14_in_site/4'>Cortistatin-14</scene>, specifically, interacts with sub-pocket 1 through LYS-3 and sub-pocket 2 through LYS-8. | ||
| + | |||
| + | |||
== Function == | == Function == | ||
| - | GPCRs undergo a transmembrane protein conformational change upon ligand binding. This signal is then transduced to the G-protein allowing for downstream responses. | + | GPCRs undergo a transmembrane protein conformational change upon ligand binding. This signal is then transduced to the G-protein allowing for downstream responses due to <scene name='90/904306/Interface_2/1'>interactions</scene> between the alpha subunit of the G-protein and the transmembrane protein. |
=== Before Activation === | === Before Activation === | ||
| - | The culmination of different motifs observed in MRGPRX2 compared to other class A GPCRs leads to external membrane ligand binding.The MRGPRX2 GPCR undergoes a much smaller conformational change upon ligand binding compared to other Class A GPCRs due to surface level binding versus deep helix binding. This contrast is represented in the photo below to show the unbound and bound transmembrane proteins of MRGPRX2 and 5-HT2A. | + | The culmination of different motifs observed in MRGPRX2 compared to other class A GPCRs leads to external membrane ligand binding. The MRGPRX2 GPCR undergoes a much smaller conformational change upon ligand binding compared to other Class A GPCRs due to surface level binding versus deep helix binding. This contrast is represented in the photo below to show the unbound and bound transmembrane proteins of MRGPRX2 and 5-HT2A. |
| - | [[Image:Screen Shot 2022-03-282 at 6.59.44 PM.png|500px|center|thumb|Overlay of unbound (transparent) and bound (opaque) transmembrane proteins of both MRGRPX2 (left) and 5-HT2AR (right).]] | + | [[Image:Screen Shot 2022-03-282 at 6.59.44 PM.png|500px|center|thumb|'''Figure 10.''' Overlay of unbound (transparent) and bound (opaque) transmembrane proteins of both MRGRPX2 (left) and 5-HT2AR (right).]] |
=== After Activation === | === After Activation === | ||
| Line 94: | Line 124: | ||
=== Clinical Relevance === | === Clinical Relevance === | ||
| + | Some cells in the human body that express the MRGPRX2 receptor include mast cells in the skin, intestines, and trachea <ref name= "Porebski">PMID: 30619367</ref>. Mast cells are involved in allergic responses by their release of histamine or other inflammatory chemicals in the body. <ref name="McNeil">PMID: 25517090</ref> These responses induce common allergic reaction and anaphylaxis symptoms, such as cutaneous itching sensations or airway constriction | ||
| + | <ref name= "Cao" /><ref name= "Yang" /><ref name="McNeil">PMID: 25517090</ref>. Mast cells can be activated by either antibodies in the human immune response or upon ligands binding to their MRGPRX2 receptors <ref name= "McNeil" />. Ligands that bind to MRGPRX2 in the natural environment to produce an allergic response include some contents of insect venom, molecules like C48/80 or other polycationic molecules. They can also respond to endogenous signaling molecules involved in inflammation pathways and immune responses such as cytokines, anaphylatoxins, or neuropeptides <ref name= "Porebski" />. Binding to MRGPRX2 triggers an intracellular signaling pathway that eventually leads to mast cells releasing their contents containing histamine, cytokines, or other inflammatory molecules that may go on to trigger the itching sensation <ref name= "Cao" /> <ref name= "Yang" />. | ||
| + | |||
| + | Many medications commonly list chronic skin itching or inflammatory responses as side effects, such as nateglinide, an anti-diabetic drug <ref name= "Cao" />. Upon analysis of some medications that list itching as side effects such as nateglinide, they structurally share many similarities to known MRGPRX2 ligands, and could therefore initiate an unwanted itch response in patients. Other drugs such as morphine or codeine also share structural features to known MRGPRX2 ligands, which can contribute to itching side effects seen in users of these drugs <ref name= "Cao" />. | ||
| + | |||
| + | Because of how some drugs initiate an unwanted itch response, | ||
| + | MRGPRX2 has been identified as a potential target for drugs either involved in the inflammatory response or to mediate drugs from causing an unwanted reaction such as [https://pubchem.ncbi.nlm.nih.gov/compound/Atracurium#section=2D-Structure Atracurium], [https://pubchem.ncbi.nlm.nih.gov/compound/441290 Rocuronium], [https://pubchem.ncbi.nlm.nih.gov/compound/2764 Ciprofloxacin], and [https://pubchem.ncbi.nlm.nih.gov/compound/149096 Levofloxacin]<ref name="Navines-Ferrer">PMID:30072729</ref>. These drugs have similarly charged regions which may interact with sub-pockets 1 and/or 2. By binding these target drugs, MRGPRX2 can elicit or prevent a response to aid with itch-related side effects. | ||
| + | |||
| + | [[Image:Screen Shot 2022-03-29 at 10.48.36 AM.png|500px|center|thumb|Schematic representation of cellular response]] | ||
== 3D Structures == | == 3D Structures == | ||
| + | [[7s8l]], MRGPRX2 Gq <br /> | ||
| + | [[7s8m]], MRGPRX2 Gi <br /> | ||
| + | [[6wha]], 5HT2AR <br /> | ||
| + | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 02:43, 12 April 2022
MRGPRX2 Human Itch G-Protein Coupled Receptor (GPCR)
| |||||||||||
References
- ↑ Hauser AS, Attwood MM, Rask-Andersen M, Schioth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017 Dec;16(12):829-842. doi: 10.1038/nrd.2017.178. Epub, 2017 Oct 27. PMID:29075003 doi:http://dx.doi.org/10.1038/nrd.2017.178
- ↑ Basith S, Cui M, Macalino SJY, Park J, Clavio NAB, Kang S, Choi S. Exploring G Protein-Coupled Receptors (GPCRs) Ligand Space via Cheminformatics Approaches: Impact on Rational Drug Design. Front Pharmacol. 2018 Mar 9;9:128. doi: 10.3389/fphar.2018.00128. eCollection, 2018. PMID:29593527 doi:http://dx.doi.org/10.3389/fphar.2018.00128
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Cao C, Kang HJ, Singh I, Chen H, Zhang C, Ye W, Hayes BW, Liu J, Gumpper RH, Bender BJ, Slocum ST, Krumm BE, Lansu K, McCorvy JD, Kroeze WK, English JG, DiBerto JF, Olsen RHJ, Huang XP, Zhang S, Liu Y, Kim K, Karpiak J, Jan LY, Abraham SN, Jin J, Shoichet BK, Fay JF, Roth BL. Structure, function and pharmacology of human itch GPCRs. Nature. 2021 Dec;600(7887):170-175. doi: 10.1038/s41586-021-04126-6. Epub 2021, Nov 17. PMID:34789874 doi:http://dx.doi.org/10.1038/s41586-021-04126-6
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 Yang F, Guo L, Li Y, Wang G, Wang J, Zhang C, Fang GX, Chen X, Liu L, Yan X, Liu Q, Qu C, Xu Y, Xiao P, Zhu Z, Li Z, Zhou J, Yu X, Gao N, Sun JP. Structure, function and pharmacology of human itch receptor complexes. Nature. 2021 Dec;600(7887):164-169. doi: 10.1038/s41586-021-04077-y. Epub 2021, Nov 17. PMID:34789875 doi:http://dx.doi.org/10.1038/s41586-021-04077-y
- ↑ Kamato D, Thach L, Bernard R, Chan V, Zheng W, Kaur H, Brimble M, Osman N, Little PJ. Structure, Function, Pharmacology, and Therapeutic Potential of the G Protein, Galpha/q,11. Front Cardiovasc Med. 2015 Mar 24;2:14. doi: 10.3389/fcvm.2015.00014. eCollection, 2015. PMID:26664886 doi:http://dx.doi.org/10.3389/fcvm.2015.00014
- ↑ Trzaskowski B, Latek D, Yuan S, Ghoshdastider U, Debinski A, Filipek S. Action of molecular switches in GPCRs--theoretical and experimental studies. Curr Med Chem. 2012;19(8):1090-109. doi: 10.2174/092986712799320556. PMID:22300046 doi:http://dx.doi.org/10.2174/092986712799320556
- ↑ Olivella M, Caltabiano G, Cordomi A. The role of Cysteine 6.47 in class A GPCRs. BMC Struct Biol. 2013 Mar 15;13:3. doi: 10.1186/1472-6807-13-3. PMID:23497259 doi:http://dx.doi.org/10.1186/1472-6807-13-3
- ↑ 8.0 8.1 Porebski G, Kwiecien K, Pawica M, Kwitniewski M. Mas-Related G Protein-Coupled Receptor-X2 (MRGPRX2) in Drug Hypersensitivity Reactions. Front Immunol. 2018 Dec 20;9:3027. doi: 10.3389/fimmu.2018.03027. eCollection, 2018. PMID:30619367 doi:http://dx.doi.org/10.3389/fimmu.2018.03027
- ↑ 9.0 9.1 9.2 McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, Dong X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015 Mar 12;519(7542):237-41. doi: 10.1038/nature14022. Epub 2014 Dec 17. PMID:25517090 doi:http://dx.doi.org/10.1038/nature14022