Sandbox Reserved 1709
From Proteopedia
| Line 5: | Line 5: | ||
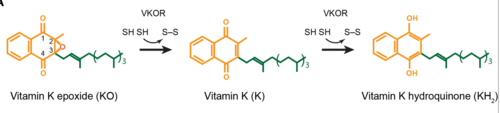
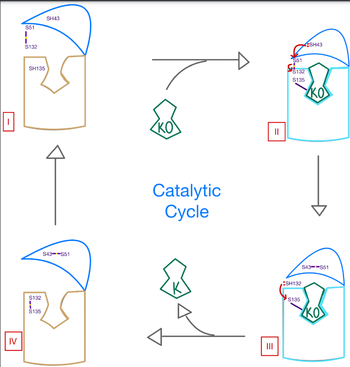
<scene name='90/906893/Vkor_structure/1'>Vitamin K epoxide reductase</scene> (VKOR) is an enzyme that, as its name implies, promotes the reduction of <scene name='90/906893/Vkor_with_ko/1'>vitamin K epoxide</scene> (KO). VKOR is a transmembrane protein spanning the endoplasmic reticulum and composed of [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2919313/ 4 transmembrane helical proteins]. One of its primary roles is to assist in blood coagulation by regenerating hydroquinone (KH2). KH2 acts as a γ-carboxylase cofactor that drives the γ-carboxylation of several coagulation factors. Structural characterization of VKOR has been difficult, though, due to its in vitro instability. Nonetheless, a near perfect atomic structure has been determined utilization anticoagulant stabilization and VKOR-like [https://pubmed.ncbi.nlm.nih.gov/33154105/ homologs]. | <scene name='90/906893/Vkor_structure/1'>Vitamin K epoxide reductase</scene> (VKOR) is an enzyme that, as its name implies, promotes the reduction of <scene name='90/906893/Vkor_with_ko/1'>vitamin K epoxide</scene> (KO). VKOR is a transmembrane protein spanning the endoplasmic reticulum and composed of [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2919313/ 4 transmembrane helical proteins]. One of its primary roles is to assist in blood coagulation by regenerating hydroquinone (KH2). KH2 acts as a γ-carboxylase cofactor that drives the γ-carboxylation of several coagulation factors. Structural characterization of VKOR has been difficult, though, due to its in vitro instability. Nonetheless, a near perfect atomic structure has been determined utilization anticoagulant stabilization and VKOR-like [https://pubmed.ncbi.nlm.nih.gov/33154105/ homologs]. | ||
| - | [[Image:VKOR_mechanism_2D.png |500 px|right| thumb]] | + | [[Image:VKOR_mechanism_2D.png |500 px|right|thumb|Figure 1. Structures of VKOR 2D manipulation for Vitamin K activation]] |
=== Author's Notes === | === Author's Notes === | ||
As previously mentioned, the VKOR structure has been challenging to qualify. Thus it is important to note that to date all VKOR structures discovered were done so from 2 methods. First, crystal structures of Human VKOR were captured with a bound substrate (KO) or vitamin K antagonist (VKA). VKA substrates utilized were anticoagulants, namely Warfarin, brodifacoum, phenindione, and chlorophacinone. Second, VKOR-like homologs, specifically isolated from the pufferfish ''Takifugu rubripes'', aided in structure classification as well. | As previously mentioned, the VKOR structure has been challenging to qualify. Thus it is important to note that to date all VKOR structures discovered were done so from 2 methods. First, crystal structures of Human VKOR were captured with a bound substrate (KO) or vitamin K antagonist (VKA). VKA substrates utilized were anticoagulants, namely Warfarin, brodifacoum, phenindione, and chlorophacinone. Second, VKOR-like homologs, specifically isolated from the pufferfish ''Takifugu rubripes'', aided in structure classification as well. | ||
Revision as of 12:21, 12 April 2022
| |||||||||||
References
1. Elshaikh, A. O., Shah, L., Joy Mathew, C., Lee, R., Jose, M. T., & Cancarevic, I. "Influence of Vitamin K on Bone Mineral Density and Osteoporosis" (2020) Cureus, 12(10), e10816. [1]
2. Guomin Shen, Weidong Cui, Qing Cao, Meng Gao, Hongli Liu, Gaigai Su, Michael L. Gross, Weikai Li. The catalytic mechanism of vitamin K epoxide reduction in a cellular environment. (2021) Journal of Biological Chemistry, Volume 296,100145. https://doi.org/10.1074/jbc.RA120.015401.
3. Li, Weikai et al. “Structure of a bacterial homologue of vitamin K epoxide reductase.” Nature vol. 463,7280 (2010): 507-12. doi:10.1038/nature08720.
4. Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2021 Jan 1;371(6524):eabc5667. doi: 10.1126/science.abc5667. Epub 2020 Nov 5. PMID: 33154105; PMCID: PMC7946407.
5. Yang W., et. al. “VKORC1 Haplotypes Are Associated With Arterial Vascular Diseases (Stroke, Coronary Heart Disease, and Aortic Dissection)” (2006) Circulation. ;113:1615–1621 [2]