Sandbox Reserved 1709
From Proteopedia
| Line 10: | Line 10: | ||
== Structural Highlights== | == Structural Highlights== | ||
| + | ===Structural Overview=== | ||
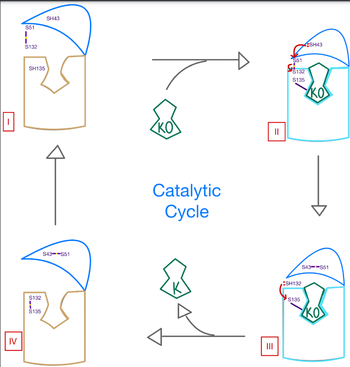
| + | VKOR has many key components of its structure that allow it to maintain proper functionality and catalytic abilities. The VKOR active site allows for specific substrate binding via many highly conserved residues that can recognize the target substrates. It works in conjunction with the cap domain, which is a helical component of the VKOR that facilitates the conformation from the open to closed conformation of the enzyme once the substrate binds. Interactions between this domain, the active site, and the bound protein are critical to achieve full activation of Vitamin K. Another important part of the structure is the anchor, which simply serves as a way to hold VKOR within the proper orientation in the cell membrane such that all enzymatic components are in correct proximity for substrate binding and catalysis. | ||
=== Active Site === | === Active Site === | ||
Within the four transmembrane helices lies the <scene name='90/906893/Active_site/4'>active site</scene>. The active site is comprised of a hydrophobic pocket containing two hydrophilic residues, A80 and Y139, that interact with substrates and ligands alike. The hydrophobic pocket provides specificity to the region while the hydrophilic residues have potential to hydrogen bond, allowing recognition and increasing specificity as well. Slightly above the active site is a crucial disulfide bridge that provides stabilization when a substrate is bound. This bridge occurs between C132 and C135, recurrent residues that continually aid in VKOR function. | Within the four transmembrane helices lies the <scene name='90/906893/Active_site/4'>active site</scene>. The active site is comprised of a hydrophobic pocket containing two hydrophilic residues, A80 and Y139, that interact with substrates and ligands alike. The hydrophobic pocket provides specificity to the region while the hydrophilic residues have potential to hydrogen bond, allowing recognition and increasing specificity as well. Slightly above the active site is a crucial disulfide bridge that provides stabilization when a substrate is bound. This bridge occurs between C132 and C135, recurrent residues that continually aid in VKOR function. | ||
| - | The active site plays a vital role in binding of any substrate or ligand to the VKOR. Upon binding, the VKOR will transition into a <scene name='90/906893/Closed_conformation/4'>closed conformation</scene> that will allow its catalytic mechanism to commence. | + | The active site plays a vital role in binding of any substrate or ligand to the VKOR. Upon binding, the VKOR will transition into a <scene name='90/906893/Closed_conformation/4'>closed conformation</scene> that will allow its catalytic mechanism to commence. |
| - | + | ||
| - | + | ||
=== Cap Domain === | === Cap Domain === | ||
A key part of VKOR is the function of the <scene name='90/904314/ cap_domain/3'>Cap Domain</scene>, which is located right above the helices of VKOR towards the intracellular part of the membrane. The cap has a helical shape and is located in close proximity to two other domains: the Anchor domain and beta hairpin. This combination of domains help to maintain the proper orientation in the membrane. The cap domain assists with activating Vitamin K as it induces the structural change of VKOR from the open conformation to the closed conformation upon substrate binding. Cap rearrangement and transition to the closed conformation initiates a domino effect through the [https://reader.elsevier.com/reader/sd/pii/S0021925820001386?token=9F8E1964241D20488CA55E035D35D9A5D650A7B3FDAD9A5579598A8DC00127539BE71CF1785B117102144AC1F41ABB6C&originRegion=us-east-1&originCreation=20220329001707/ catalytic mechanism]. The cap domain has critical interactions that stabilize the closed conformation including a <scene name='90/904314/Disulfide_bridge_stabilization/3'> disulfide bridge</scene> between C43 and C51, and polar interactions from D44. Catalytic cysteines help facilitate this transition from the open conformation to the closed conformation in order to assist with activation of Vitamin K. | A key part of VKOR is the function of the <scene name='90/904314/ cap_domain/3'>Cap Domain</scene>, which is located right above the helices of VKOR towards the intracellular part of the membrane. The cap has a helical shape and is located in close proximity to two other domains: the Anchor domain and beta hairpin. This combination of domains help to maintain the proper orientation in the membrane. The cap domain assists with activating Vitamin K as it induces the structural change of VKOR from the open conformation to the closed conformation upon substrate binding. Cap rearrangement and transition to the closed conformation initiates a domino effect through the [https://reader.elsevier.com/reader/sd/pii/S0021925820001386?token=9F8E1964241D20488CA55E035D35D9A5D650A7B3FDAD9A5579598A8DC00127539BE71CF1785B117102144AC1F41ABB6C&originRegion=us-east-1&originCreation=20220329001707/ catalytic mechanism]. The cap domain has critical interactions that stabilize the closed conformation including a <scene name='90/904314/Disulfide_bridge_stabilization/3'> disulfide bridge</scene> between C43 and C51, and polar interactions from D44. Catalytic cysteines help facilitate this transition from the open conformation to the closed conformation in order to assist with activation of Vitamin K. | ||
Revision as of 12:52, 12 April 2022
| |||||||||||
References
1. Elshaikh, A. O., Shah, L., Joy Mathew, C., Lee, R., Jose, M. T., & Cancarevic, I. "Influence of Vitamin K on Bone Mineral Density and Osteoporosis" (2020) Cureus, 12(10), e10816. [1]
2. Guomin Shen, Weidong Cui, Qing Cao, Meng Gao, Hongli Liu, Gaigai Su, Michael L. Gross, Weikai Li. The catalytic mechanism of vitamin K epoxide reduction in a cellular environment. (2021) Journal of Biological Chemistry, Volume 296,100145. https://doi.org/10.1074/jbc.RA120.015401.
3. Li, Weikai et al. “Structure of a bacterial homologue of vitamin K epoxide reductase.” Nature vol. 463,7280 (2010): 507-12. doi:10.1038/nature08720.
4. Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2021 Jan 1;371(6524):eabc5667. doi: 10.1126/science.abc5667. Epub 2020 Nov 5. PMID: 33154105; PMCID: PMC7946407.
5. Yang W., et. al. “VKORC1 Haplotypes Are Associated With Arterial Vascular Diseases (Stroke, Coronary Heart Disease, and Aortic Dissection)” (2006) Circulation. ;113:1615–1621 [2]