We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1705
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
=== Ligand Binding=== | === Ligand Binding=== | ||
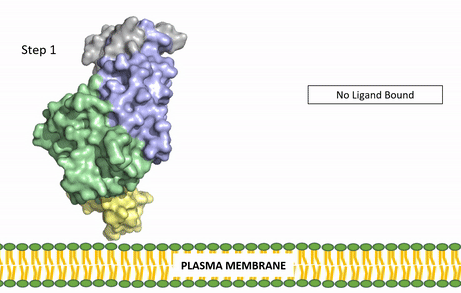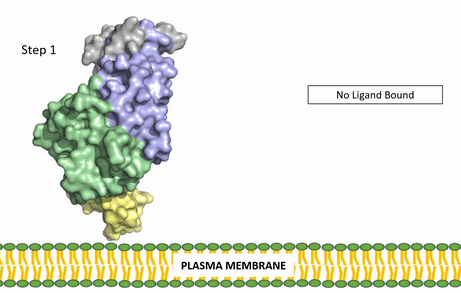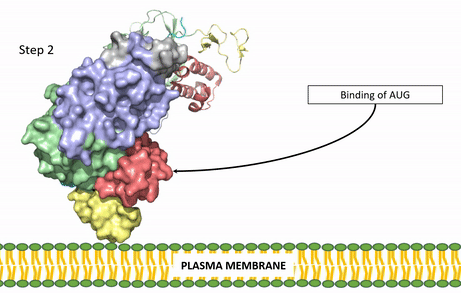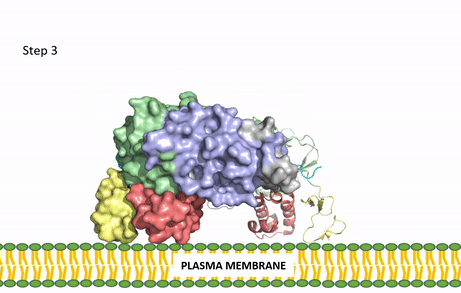
| - | The ligands recognized by anaplastic lymphoma kinase are FAM150 in a monomeric fashion and <scene name='90/904310/Ligand/1'>AUG</scene> in a dimeric fashion. It's biologically preferred ligand is AUG, a 128 monomer peptide ligand. The binding of ALK to it's ligand results in homodimerization and a conformational change. Prior to the ligand binding to anaplastic lymphoma kinase, the extracellular domain is oriented vertically and perpendicularly to the plasma membrane. Once the ligand is <scene name='90/904310/Dimer_ligand_complex/3'>bound</scene>, ALK undergoes a conformational change and folds over so that the positively charged residues on the portion of the protein previously oriented vertically is now interacting with the negatively charged residues on the plasma membrane. The residues of ALK and it's ligand interact through the formation of <scene name='90/904310/Dimer-ligand-interface/4'>salt bridges</scene>. This conformational change via ligand binding induces the auto-activation of the kinase domain, in which the domains use the tyrosine phosphorylation mechanism to phosphorylate tyrosine residues on the opposite monomer. [[Image: | + | The ligands recognized by anaplastic lymphoma kinase are FAM150 in a monomeric fashion and <scene name='90/904310/Ligand/1'>AUG</scene> in a dimeric fashion. It's biologically preferred ligand is AUG, a 128 monomer peptide ligand. The binding of ALK to it's ligand results in homodimerization and a conformational change. Prior to the ligand binding to anaplastic lymphoma kinase, the extracellular domain is oriented vertically and perpendicularly to the plasma membrane. Once the ligand is <scene name='90/904310/Dimer_ligand_complex/3'>bound</scene>, ALK undergoes a conformational change and folds over so that the positively charged residues on the portion of the protein previously oriented vertically is now interacting with the negatively charged residues on the plasma membrane. The residues of ALK and it's ligand interact through the formation of <scene name='90/904310/Dimer-ligand-interface/4'>salt bridges</scene>. This conformational change via ligand binding induces the auto-activation of the kinase domain, in which the domains use the tyrosine phosphorylation mechanism to phosphorylate tyrosine residues on the opposite monomer. [[Image:ALK Conformational Change Gif.gif|850 px|left|thumb|Figure 2: Gif-image of the conformational change occurring in the extracellular region of Anaplastic Lymphoma Kinase once the AUG ligand has bound to the ligand binding site. This change is stabilized through contacts of the AUG and the plasma membrane. The video was made using stop motion animation techniques, then converted to gif format using EZgif.]] |
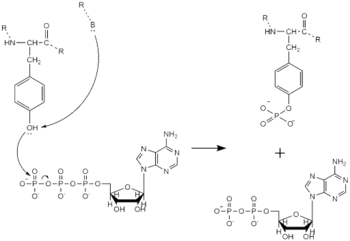
==== Tyrosine Phosphorylation Mechanism ==== | ==== Tyrosine Phosphorylation Mechanism ==== | ||
Revision as of 14:48, 12 April 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
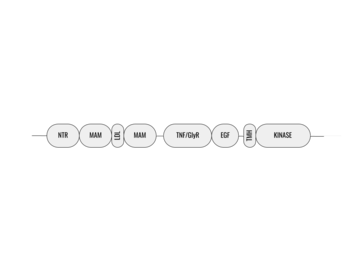
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Reshetnyak AV, Rossi P, Myasnikov AG, Sowaileh M, Mohanty J, Nourse A, Miller DJ, Lax I, Schlessinger J, Kalodimos CG. Mechanism for the activation of the anaplastic lymphoma kinase receptor. Nature. 2021 Dec;600(7887):153-157. doi: 10.1038/s41586-021-04140-8. Epub 2021, Nov 24. PMID:34819673 doi:http://dx.doi.org/10.1038/s41586-021-04140-8
- ↑ Palmer RH, Vernersson E, Grabbe C, Hallberg B. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J. 2009 May 27;420(3):345-61. doi: 10.1042/BJ20090387. PMID:19459784 doi:http://dx.doi.org/10.1042/BJ20090387
- ↑ Li T, Stayrook SE, Tsutsui Y, Zhang J, Wang Y, Li H, Proffitt A, Krimmer SG, Ahmed M, Belliveau O, Walker IX, Mudumbi KC, Suzuki Y, Lax I, Alvarado D, Lemmon MA, Schlessinger J, Klein DE. Structural basis for ligand reception by anaplastic lymphoma kinase. Nature. 2021 Dec;600(7887):148-152. doi: 10.1038/s41586-021-04141-7. Epub 2021, Nov 24. PMID:34819665 doi:http://dx.doi.org/10.1038/s41586-021-04141-7
- ↑ Huang H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int J Mol Sci. 2018 Nov 2;19(11). pii: ijms19113448. doi: 10.3390/ijms19113448. PMID:30400214 doi:http://dx.doi.org/10.3390/ijms19113448
- ↑ Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer. 2013 Oct;13(10):685-700. doi: 10.1038/nrc3580. PMID:24060861 doi:http://dx.doi.org/10.1038/nrc3580
- ↑ Selander-Sunnerhagen M, Ullner M, Persson E, Teleman O, Stenflo J, Drakenberg T. How an epidermal growth factor (EGF)-like domain binds calcium. High resolution NMR structure of the calcium form of the NH2-terminal EGF-like domain in coagulation factor X. J Biol Chem. 1992 Sep 25;267(27):19642-9. PMID:1527084
- ↑ Lewis RT, Bode CM, Choquette D, Potashman M, Romero K, Stellwagen JC, Teffera Y, Moore E, Whittington DA, Chen H, Epstein LF, Emkey R, Andrews PS, Yu V, Saffran DC, Xu M, Drew AE, Merkel P, Szilvassy S, Brake RL. The discovery and optimization of a novel class of potent, selective and orally bioavailable Anaplastic Lymphoma Kinase (ALK) Inhibitors with potential utility for the treatment of cancer. J Med Chem. 2012 Jun 26. PMID:22734674 doi:10.1021/jm3005866
- ↑ 8.0 8.1 Sahu A, Prabhash K, Noronha V, Joshi A, Desai S. Crizotinib: A comprehensive review. South Asian J Cancer. 2013 Apr;2(2):91-7. doi: 10.4103/2278-330X.110506. PMID:24455567 doi:http://dx.doi.org/10.4103/2278-330X.110506
- ↑ 9.0 9.1 Wang Q, Zorn JA, Kuriyan J. A structural atlas of kinases inhibited by clinically approved drugs. Methods Enzymol. 2014;548:23-67. doi: 10.1016/B978-0-12-397918-6.00002-1. PMID:25399641 doi:http://dx.doi.org/10.1016/B978-0-12-397918-6.00002-1
Student Contributors
- Kaylin Todor
- Rebekah White