We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1712
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
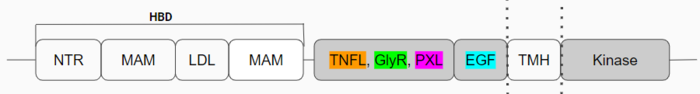
The domains that aren't shown in Figure 2 but are shown in the domain map (Figure 1) also make up the monomer. The heparin binding domains (HBDs), are at the N-terminal end of the monomer. Heparin has been found to be a possible activating ligand of ALK.<ref>DOI: 10.1126/scisignal.2005916</ref> The transmembrane domain (TMH) contains the residues of ALK that are located within the membrane. The kinase domain is the intracellular portion of ALK that contains the Tyr residues which are auto-phosphorylated when ALK is activated, initiating a signaling cascade. <ref>DOI: 10.1038/s41586-021-04141-7</ref> | The domains that aren't shown in Figure 2 but are shown in the domain map (Figure 1) also make up the monomer. The heparin binding domains (HBDs), are at the N-terminal end of the monomer. Heparin has been found to be a possible activating ligand of ALK.<ref>DOI: 10.1126/scisignal.2005916</ref> The transmembrane domain (TMH) contains the residues of ALK that are located within the membrane. The kinase domain is the intracellular portion of ALK that contains the Tyr residues which are auto-phosphorylated when ALK is activated, initiating a signaling cascade. <ref>DOI: 10.1038/s41586-021-04141-7</ref> | ||
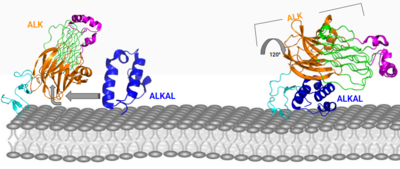
| - | [[Image:Conformational change 2D.png|400 px| | + | [[Image:Conformational change 2D.png|400 px|left|thumb|Figure 3: ALK-ALKAL complex, showing the conformation change of ALK from the binding of ALKAL. [https://www.rcsb.org/structure/7N00 PDB: 7N00]]] |
===Conformational Change=== | ===Conformational Change=== | ||
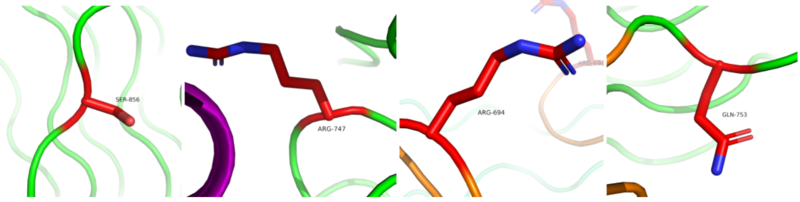
The anaplastic lymphoma kinase activating ligand (ALKAL) binds to the <scene name='90/904317/Alk-alkal_binding_surface/1'>binding surface</scene> on the ALK at the TNFL domain. This induces a conformational change which allows for the PXL and the GlyR domains to hinge forward.<ref>DOI: 10.1038/s41586-021-04140-8</ref> (Figure 3) ALK's TNFL has <scene name='90/904317/Binding_surface_with_residues/1'>residues</scene> E978, E974, E859, and Y966 that form salt bridges with R123, R133, R136, R140, and R117 on ALKAL that allow for activation, leading to <scene name='90/904317/Dimer_full_colored/5'>dimerization</scene> of two ALK-ALKAL monomers. | The anaplastic lymphoma kinase activating ligand (ALKAL) binds to the <scene name='90/904317/Alk-alkal_binding_surface/1'>binding surface</scene> on the ALK at the TNFL domain. This induces a conformational change which allows for the PXL and the GlyR domains to hinge forward.<ref>DOI: 10.1038/s41586-021-04140-8</ref> (Figure 3) ALK's TNFL has <scene name='90/904317/Binding_surface_with_residues/1'>residues</scene> E978, E974, E859, and Y966 that form salt bridges with R123, R133, R136, R140, and R117 on ALKAL that allow for activation, leading to <scene name='90/904317/Dimer_full_colored/5'>dimerization</scene> of two ALK-ALKAL monomers. | ||
Revision as of 19:56, 12 April 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
Anaplastic Lymphoma Kinase receptor
| |||||||||||
References
- ↑ Huang H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int J Mol Sci. 2018 Nov 2;19(11). pii: ijms19113448. doi: 10.3390/ijms19113448. PMID:30400214 doi:http://dx.doi.org/10.3390/ijms19113448
- ↑ Murray PB, Lax I, Reshetnyak A, Ligon GF, Lillquist JS, Natoli EJ Jr, Shi X, Folta-Stogniew E, Gunel M, Alvarado D, Schlessinger J. Heparin is an activating ligand of the orphan receptor tyrosine kinase ALK. Sci Signal. 2015 Jan 20;8(360):ra6. doi: 10.1126/scisignal.2005916. PMID:25605972 doi:http://dx.doi.org/10.1126/scisignal.2005916
- ↑ Li T, Stayrook SE, Tsutsui Y, Zhang J, Wang Y, Li H, Proffitt A, Krimmer SG, Ahmed M, Belliveau O, Walker IX, Mudumbi KC, Suzuki Y, Lax I, Alvarado D, Lemmon MA, Schlessinger J, Klein DE. Structural basis for ligand reception by anaplastic lymphoma kinase. Nature. 2021 Dec;600(7887):148-152. doi: 10.1038/s41586-021-04141-7. Epub 2021, Nov 24. PMID:34819665 doi:http://dx.doi.org/10.1038/s41586-021-04141-7
- ↑ Reshetnyak AV, Rossi P, Myasnikov AG, Sowaileh M, Mohanty J, Nourse A, Miller DJ, Lax I, Schlessinger J, Kalodimos CG. Mechanism for the activation of the anaplastic lymphoma kinase receptor. Nature. 2021 Dec;600(7887):153-157. doi: 10.1038/s41586-021-04140-8. Epub 2021, Nov 24. PMID:34819673 doi:http://dx.doi.org/10.1038/s41586-021-04140-8
- ↑ De Munck S, Provost M, Kurikawa M, Omori I, Mukohyama J, Felix J, Bloch Y, Abdel-Wahab O, Bazan JF, Yoshimi A, Savvides SN. Structural basis of cytokine-mediated activation of ALK family receptors. Nature. 2021 Oct 13. pii: 10.1038/s41586-021-03959-5. doi:, 10.1038/s41586-021-03959-5. PMID:34646012 doi:http://dx.doi.org/10.1038/s41586-021-03959-5
Student Contributors
- Drew Peters
- Hillary Kulavic