We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1712
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
== Structure & Function == | == Structure & Function == | ||
===Domains=== | ===Domains=== | ||
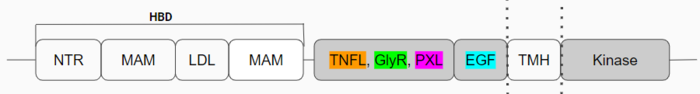
| - | [[Image:Updated schematic.png|700 px| | + | [[Image:Updated schematic.png|700 px|center|thumb|Figure 1: Anaplastic Lymphoma Kinase and its domains.]] |
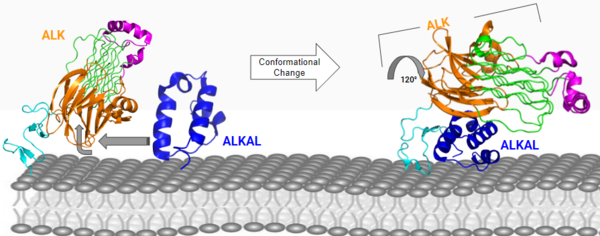
The region from NTR to the MAM is the Heparin Binding Domain. The TNFL-PXL are the extracellular domains and the EGF is the domain that binds the extracellular region with the extracellular region of the transmembrane. The TMH is the transmembrane domain. The kinase domain is the intracellular portion of the ALK. The extracellular portion of ALK has an inactive state, which is its monomerized form, and an active dimerized state with its ligands bound. The monomer is shown in Figure 2, which has many different domains (Figure 1). The growth factor-like domain (EGF) connects the extracellular domains to the transmembrane domain (cyan). The tumor necrosis factor-like domain (TNFL) has a beta-sandwich structure that provides important residues that act as the binding surface for the ligand (orange). The glycine-rich domain (GlyR) contains 14 rare polyglycine helices that are hydrogen-bound to each other (green). The <scene name='90/904317/Glycinerichdomain/1'>hexagonal orientation</scene> of these rare helices create a very rigid structure that is important for ALK function. The polyglycine extension loop (PXL) connects two of these polyglycine helices (pink). | The region from NTR to the MAM is the Heparin Binding Domain. The TNFL-PXL are the extracellular domains and the EGF is the domain that binds the extracellular region with the extracellular region of the transmembrane. The TMH is the transmembrane domain. The kinase domain is the intracellular portion of the ALK. The extracellular portion of ALK has an inactive state, which is its monomerized form, and an active dimerized state with its ligands bound. The monomer is shown in Figure 2, which has many different domains (Figure 1). The growth factor-like domain (EGF) connects the extracellular domains to the transmembrane domain (cyan). The tumor necrosis factor-like domain (TNFL) has a beta-sandwich structure that provides important residues that act as the binding surface for the ligand (orange). The glycine-rich domain (GlyR) contains 14 rare polyglycine helices that are hydrogen-bound to each other (green). The <scene name='90/904317/Glycinerichdomain/1'>hexagonal orientation</scene> of these rare helices create a very rigid structure that is important for ALK function. The polyglycine extension loop (PXL) connects two of these polyglycine helices (pink). | ||
Revision as of 20:15, 12 April 2022
Anaplastic Lymphoma Kinase receptor
| |||||||||||
References
- ↑ Huang H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int J Mol Sci. 2018 Nov 2;19(11). pii: ijms19113448. doi: 10.3390/ijms19113448. PMID:30400214 doi:http://dx.doi.org/10.3390/ijms19113448
- ↑ Huang H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int J Mol Sci. 2018 Nov 2;19(11). pii: ijms19113448. doi: 10.3390/ijms19113448. PMID:30400214 doi:http://dx.doi.org/10.3390/ijms19113448
- ↑ Murray PB, Lax I, Reshetnyak A, Ligon GF, Lillquist JS, Natoli EJ Jr, Shi X, Folta-Stogniew E, Gunel M, Alvarado D, Schlessinger J. Heparin is an activating ligand of the orphan receptor tyrosine kinase ALK. Sci Signal. 2015 Jan 20;8(360):ra6. doi: 10.1126/scisignal.2005916. PMID:25605972 doi:http://dx.doi.org/10.1126/scisignal.2005916
- ↑ Li T, Stayrook SE, Tsutsui Y, Zhang J, Wang Y, Li H, Proffitt A, Krimmer SG, Ahmed M, Belliveau O, Walker IX, Mudumbi KC, Suzuki Y, Lax I, Alvarado D, Lemmon MA, Schlessinger J, Klein DE. Structural basis for ligand reception by anaplastic lymphoma kinase. Nature. 2021 Dec;600(7887):148-152. doi: 10.1038/s41586-021-04141-7. Epub 2021, Nov 24. PMID:34819665 doi:http://dx.doi.org/10.1038/s41586-021-04141-7
- ↑ Reshetnyak AV, Rossi P, Myasnikov AG, Sowaileh M, Mohanty J, Nourse A, Miller DJ, Lax I, Schlessinger J, Kalodimos CG. Mechanism for the activation of the anaplastic lymphoma kinase receptor. Nature. 2021 Dec;600(7887):153-157. doi: 10.1038/s41586-021-04140-8. Epub 2021, Nov 24. PMID:34819673 doi:http://dx.doi.org/10.1038/s41586-021-04140-8
- ↑ De Munck S, Provost M, Kurikawa M, Omori I, Mukohyama J, Felix J, Bloch Y, Abdel-Wahab O, Bazan JF, Yoshimi A, Savvides SN. Structural basis of cytokine-mediated activation of ALK family receptors. Nature. 2021 Oct 13. pii: 10.1038/s41586-021-03959-5. doi:, 10.1038/s41586-021-03959-5. PMID:34646012 doi:http://dx.doi.org/10.1038/s41586-021-03959-5
Student Contributors
- Drew Peters
- Hillary Kulavic