This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Kia Yang/sandbox
From Proteopedia
| Line 1: | Line 1: | ||
This page is being worked on during the Spring 2022 semester. | This page is being worked on during the Spring 2022 semester. | ||
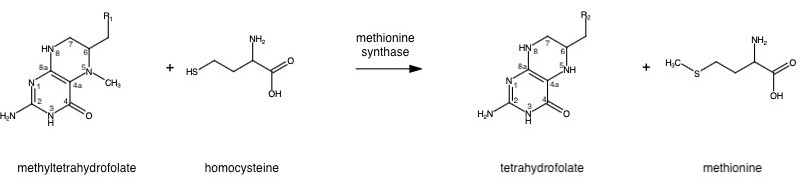
| - | '''Methionine synthase''' (abbrev. MS; EC: 2.1.1.13) is a B12-dependent enzyme | + | '''Methionine synthase''' (abbrev. MS; EC: 2.1.1.13) is a B12-dependent enzyme that catalyzes the methylation of homocysteine to methionine. This enzyme is a critical part of the one-carbon metabolism cycle as methionine is an essential amino acid required by our bodies for healthy cell and tissue growth. It is not naturally derived in our bodies, thus requiring the conversion of homocysteine, obtained from our diet, to methionine. MS mutations and B-12 deficiencies are associated with serious health conditions such as birth abnormalities and anemia. |
| Line 8: | Line 8: | ||
[[Image:Overall.jpeg]] | [[Image:Overall.jpeg]] | ||
| - | The change from homocysteine to methionine is an SN2 reaction, as seen above, where the methyl group on N-5 from methyltetrahydrofolate (MTHF), is donated. MTHF is a product of | + | The change from homocysteine to methionine is an SN2 reaction, as seen above, where the methyl group on N-5 from methyltetrahydrofolate (MTHF), is donated. MTHF is a product of methylenetetrahydrofolate reductase (MTHFR) from the folate cycle [link Shaylie's page here]. This is a complex reaction as tetrahydrofolate (THF), the product, is a poor leaving group and requires a "super nucleophile", vitamin B12 Cob(I)alamin, to carry out the reaction<ref name="Kung et al">DOI: 10.1038/nature10916</ref>; the methyl carrier. |
| + | |||
| + | MS undergoes two cycles: catalytic and reductive reactivation cycles. | ||
| + | |||
| + | Catalytic Cycle: | ||
| + | Cob(I)alamin is required in order to carry through with the complex SN2 reaction of breaking the bond between THF and the methyl group. | ||
| + | |||
| + | Co(I) - reactive but unstable, high energy | ||
| + | |||
| + | Reactivation Cycle: | ||
| + | In aerobic conditions, Cob(I)alamin occasionally undergoes oxidation leading to an inactive Cob(II)alamin enzyme. This is regulated by reductive methylation to ctivate Cob(I)alamin with Flavodoxin as an electron donor, and subsequently regenerating Me-Cob(I)alamin with SAM as the methyl donor. | ||
| + | |||
== Relevance == | == Relevance == | ||
| Line 33: | Line 44: | ||
Catalytic Cycle: | Catalytic Cycle: | ||
Cobalt in the +1 oxidation state is required in order to carry through with the complex SN2 reaction of breaking the bond between THF and the methyl group. | Cobalt in the +1 oxidation state is required in order to carry through with the complex SN2 reaction of breaking the bond between THF and the methyl group. | ||
| - | |||
| - | Co(I) - reactive but unstable, high energy | ||
Reactivation Cycle: | Reactivation Cycle: | ||
Current revision
This page is being worked on during the Spring 2022 semester.
Methionine synthase (abbrev. MS; EC: 2.1.1.13) is a B12-dependent enzyme that catalyzes the methylation of homocysteine to methionine. This enzyme is a critical part of the one-carbon metabolism cycle as methionine is an essential amino acid required by our bodies for healthy cell and tissue growth. It is not naturally derived in our bodies, thus requiring the conversion of homocysteine, obtained from our diet, to methionine. MS mutations and B-12 deficiencies are associated with serious health conditions such as birth abnormalities and anemia.
Contents |
Function
The change from homocysteine to methionine is an SN2 reaction, as seen above, where the methyl group on N-5 from methyltetrahydrofolate (MTHF), is donated. MTHF is a product of methylenetetrahydrofolate reductase (MTHFR) from the folate cycle [link Shaylie's page here]. This is a complex reaction as tetrahydrofolate (THF), the product, is a poor leaving group and requires a "super nucleophile", vitamin B12 Cob(I)alamin, to carry out the reaction[1]; the methyl carrier.
MS undergoes two cycles: catalytic and reductive reactivation cycles.
Catalytic Cycle: Cob(I)alamin is required in order to carry through with the complex SN2 reaction of breaking the bond between THF and the methyl group.
Co(I) - reactive but unstable, high energy
Reactivation Cycle: In aerobic conditions, Cob(I)alamin occasionally undergoes oxidation leading to an inactive Cob(II)alamin enzyme. This is regulated by reductive methylation to ctivate Cob(I)alamin with Flavodoxin as an electron donor, and subsequently regenerating Me-Cob(I)alamin with SAM as the methyl donor.
Relevance
MS mutations and B-12 deficiencies can result in diseases[1].
Structural highlights
| |||||||||||
References
- ↑ 1.0 1.1 Kung Y, Ando N, Doukov TI, Blasiak LC, Bender G, Seravalli J, Ragsdale SW, Drennan CL. Visualizing molecular juggling within a B(12)-dependent methyltransferase complex. Nature. 2012 Mar 14. doi: 10.1038/nature10916. PMID:22419154 doi:10.1038/nature10916
- ↑ 2.0 2.1 Bandarian V, Pattridge KA, Lennon BW, Huddler DP, Matthews RG, Ludwig ML. Domain alternation switches B(12)-dependent methionine synthase to the activation conformation. Nat Struct Biol. 2002 Jan;9(1):53-6. PMID:11731805 doi:10.1038/nsb738
- ↑ Barra L, Fontenelle C, Ermel G, Trautwetter A, Walker GC, Blanco C. Interrelations between glycine betaine catabolism and methionine biosynthesis in Sinorhizobium meliloti strain 102F34. J Bacteriol. 2006 Oct;188(20):7195-204. doi: 10.1128/JB.00208-06. PMID:17015658 doi:http://dx.doi.org/10.1128/JB.00208-06