ALK</scene>' size='350' frame='true'side='right' caption='Anaplastic Lymphoma Kinase receptor PDB code: 7n00' scene=’’>
Background
The anaplastic lymphoma kinase (ALK) was first discovered in 1994 as a tyrosine kinase in anaplastic large-cell lymphoma (ALCL) cells.[1] The specific type of tyrosine kinase ALK is classified as is a receptor tyrosine kinase (RTK) and like other RTKs, it's an integral protein with extracellular and intracellular domains and is involved in transmembrane signaling and communication within the cell. ALK is commonly expressed in the development of the nervous system. Anaplastic lymphoma kinase receptor (ALKr) is the extracellular portion of the RTK that includes a binding surface for a ligand to bind. When the ALK activating ligand (ALKAL) binds to ALKr, this causes a conformational change of ALK, allowing two ALK-ALKAL complexes to interact with each other, which will then allow intracellular kinase domain of ALK to phosphorylate a tyrosine residue on a downstream enzyme, which will activate this enzyme and activate a signaling cascade. Abnormal forms of ALK are closely related to the formation of several cancers. [2]
Structure & Function
Domains
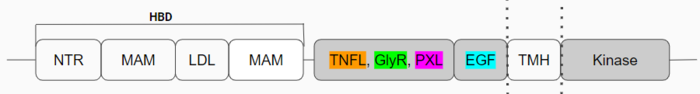
Figure 1: Anaplastic Lymphoma Kinase and its domains.
The region from NTR (N-terminal region) to the MAM is the Heparin Binding Domain. The TNFL through the PXL (polyglycine extension loop) are the extracellular domains and the EGF is the domain that binds to the TMH (transmembrane region) region in the membrane. The kinase domain is the intracellular portion of the ALK and the structure has not been discovered. The only structures that have been fully discovered are in color (Figure 1). The growth factor-like domain (EGF) connects the extracellular domains to the transmembrane domain (cyan). The tumor necrosis factor-like domain (TNFL) has a beta-sandwich structure that provides important residues that act as the binding surface for the ligand (orange). The glycine-rich domain (GlyR) contains 14 rare polyglycine helices that are hydrogen-bound to each other (green). The extracellular portion of ALK has an inactive state , and upon ligand binding, ALK transitions to an active state. The monomer has many different domains (Figure 1). The of these rare helices create a rigid structure that is important for ALK function. The domain functions as a scaffold to anchor the ligand-binding site on the TNF-like domain and the overall dimerization of the three-helix bundle. The polyglycine extension loop (PXL) connects two of these polyglycine helices (pink).
The domains that aren't shown in Figure 2 but are shown in the domain map (Figure 1) also make up the monomer. The heparin binding domains (HBDs), are at the N-terminal end of the monomer. Heparin has been found to be a possible activating ligand of ALK.[3] The transmembrane domain (TMH) contains the residues of ALK that are located within the membrane. The kinase domain is the intracellular portion of ALK that contains the Tyr residues which are auto-phosphorylated when ALK is activated, initiating a signaling cascade. [4]
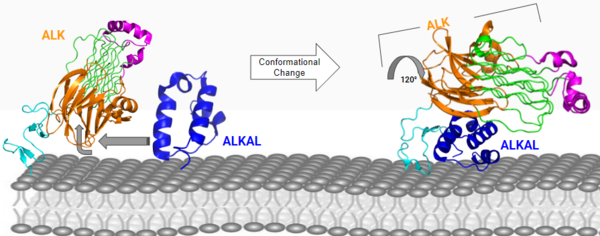
Figure 3: ALK-ALKAL complex, showing the conformation change of ALK from the binding of ALKAL.
PDB: 7N00 Membrane Guidance of ALKAL to ALK
The first step to the activation of ALK is to bind the ALK activating ligand (ALKAL) to ALKr. ALKAL is a triple alpha-helix polypeptide structure that signals for a conformational change of ALK. What allows ALKAL to interact with ALKr is the cell membrane. The negatively charged phosphate groups on the cell membrane interact with a highly conserved positively charged on ALKAL that faces the membrane. These (7MZZ) guide ALKAL to ALKr and correctly positions ALKAL for its binding surface to face ALKr's binding surface, which allows for a more favorable interaction.
Conformational Change
ALKAL to ALKr at the TNFL domain, which has important negatively charged residues that form with positively charged residues on ALKAL. These bonds initiate the conformational change, as these residues can only come into close proximity with each other if the conformational change occurs. The PXL and GlyR domains hinge forward when the change is initiated[5] (Figure 2). Glu978, Glu974, Glu859, and Tyr966 are the residues of ALKr that form these bonds with Arg123, Arg133, Arg136, Arg140, and Arg117 of ALKAL. Once the ALK-ALKAL complex is formed, the of two ALK-ALKAL complexes occurs. The main driving force of the interaction between two ALK-ALKAL complexes that dimerize are hydrophobic interactions of the PXL loop of one ALKr with the other complex's ALKAL and TNFL domain of ALKr. This dimer of two ALK-ALKAL complexes is the active form of ALK, and it is now able to perform its main function of phosphorylation.
Role of Activated ALK
Once the ALKAL binds with ALK and dimerizes with another ALK-ALKAL complex, this activated conformation also initiates a conformational change of the intracellular kinase domain of ALK. This causes an autophosphorylation of several tyrosine residues of this domain, activating a signaling cascade with its kinase activity.
Disease
There are many that would cause constitutive receptor activation, enhancement between the interaction of receptors or stabilization of active receptors are known to relate to oncogenic potentials (Figure 4). The that is mutated to arginine is known to be a gain-of-function in lung adenocarcinoma which can lead to constitutive activation of ALK (Fig. 4a). The changing to arginine could cause possible oncogenic potentials which are not specified yet (Fig. 4b). The F856S and R753Q mutations are known to increase cytokine-dependent cell proliferation in certain cells. [6]

Figure 4: Mutated residues on ALK that contribute to stabilization of the active state of ALK, leading to many types of cancers. From left to right: F856S, G747R, H694R, R753Q
PDB: 7N00


