This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1705
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
=== Ligand Binding=== | === Ligand Binding=== | ||
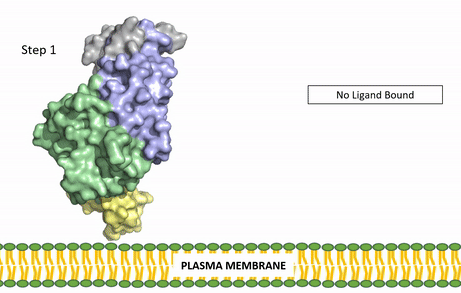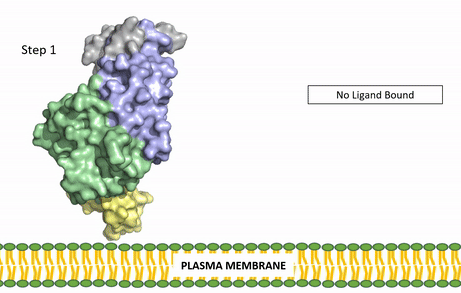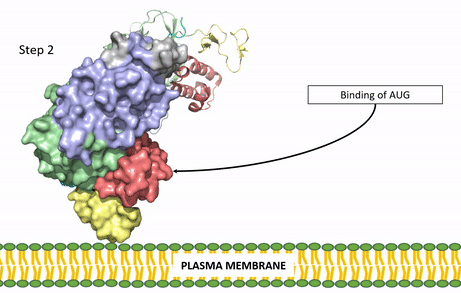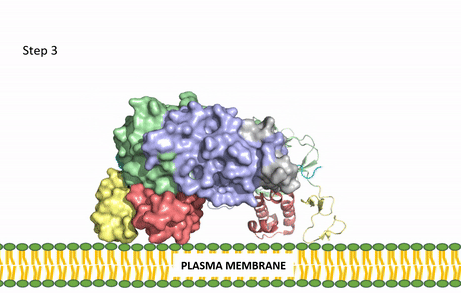
| - | [[Image:ALK Conformational Change Gif.gif|850 px| | + | [[Image:ALK Conformational Change Gif.gif|850 px|right|thumb|Figure 3: Gif-image of the conformational change occurring in the extracellular region of Anaplastic Lymphoma Kinase once the AUG ligand has bound to the ligand-binding site. This change is stabilized through contacts of the AUG and the plasma membrane. The video was made using stop motion animation techniques, then converted to gif format using EZgif.]] |
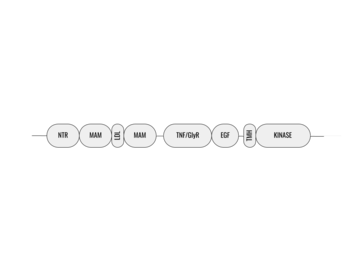
| - | The ligands recognized by anaplastic lymphoma kinase are FAM150 in a monomeric fashion and <scene name='90/904310/Ligand/2'>AUG</scene> in a dimeric fashion <ref name="Li">PMID:34819665</ref> . Its biologically preferred ligand is AUG, a 128 monomer peptide ligand. FAM150 is a structural ortholog of AUG. The binding of ALK to it's ligand results in homodimerization and a conformational change. Prior to the ligand binding to anaplastic lymphoma kinase, the extracellular domain is oriented vertically and perpendicularly to the plasma membrane (Step 1, Figure | + | The ligands recognized by anaplastic lymphoma kinase are FAM150 in a monomeric fashion and <scene name='90/904310/Ligand/2'>AUG</scene> in a dimeric fashion <ref name="Li">PMID:34819665</ref> . Its biologically preferred ligand is AUG, a 128 monomer peptide ligand. FAM150 is a structural ortholog of AUG. The binding of ALK to it's ligand results in homodimerization and a conformational change. Prior to the ligand binding to anaplastic lymphoma kinase, the extracellular domain is oriented vertically and perpendicularly to the plasma membrane (Step 1, Figure 3). Once the ligand is <scene name='90/904310/Dimer_ligand_complex/5'>bound</scene> (Step 2, Figure 3), ALK undergoes a conformational change and folds over so that the positively charged residues on the portion of the protein previously oriented vertically is now interacting with the negatively charged residues on the plasma membrane (Step 3, Figure 3). The residues of ALK and it's ligand interact through the formation of <scene name='90/904310/Dimer-ligand-interface/5'>salt bridges</scene><ref name="Munck">PMID:34646012</ref>. It has been hypothesized that the GlyR region plays a role in the flexibility needed to complete the conformational change. This conformational change via ligand binding induces the auto-activation of the kinase domain, in which the domains use the tyrosine phosphorylation mechanism to phosphorylate tyrosine residues on the opposite monomer. Another well-known RTK is the insulin receptor which is activated using a similar pathway. To learn more about the larger mechanism of RTK activation, click [https://proteopedia.org/wiki/index.php/Insulin_receptor#:~:text=The%20insulin%20receptor%20binds%20the,including%20skeletal%20muscle%20and%20adipose here]. |
==== Tyrosine Phosphorylation Mechanism ==== | ==== Tyrosine Phosphorylation Mechanism ==== | ||
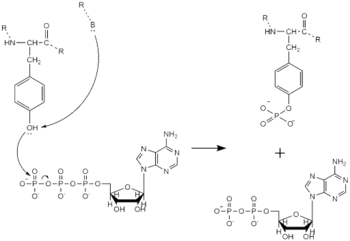
| - | Dimerization of anaplastic lymphoma kinase activates the <scene name='90/904309/Kinase_domain/2'>kinase domain</scene> of each monomer <ref name="Li">PMID:34819665</ref>. Next, the kinase domains phosphorylate the [https://en.wikipedia.org/wiki/Tyrosine_phosphorylation tyrosine residues] (Y1278, Y1282, and Y1283 <ref name="Selander-Sunnerhagen">PMID:1527084</ref>) of the opposite monomer using ATP <ref name="Munck">PMID:34646012</ref>. These phosphorylated tyrosine residues recruit signal proteins through phosphorylation. These signal proteins begin a signaling cascade by utilizing various signal pathways including the ERK, JAK, and PI3K pathways. These pathways signal for cell proliferation and survival (ex: begin transcription). The mechanism of tyrosine phosphorylation is a key step in signal transduction and regulation of enzymatic activity. This mechanism is widely used with a variety of proteins and enzymes including being used in insulin signaling. If there is a problem with this mechanism, it can lead to many ill effects as the signaling pathways instigated by this mechanism will be unable to function correctly. | + | [[Image:Tyrosine_Mechanism_Picture.png|350 px|left|thumb|Figure 4. Tyrosine phosphorylation mechanism]] |
| + | Dimerization of anaplastic lymphoma kinase activates the <scene name='90/904309/Kinase_domain/2'>kinase domain</scene> of each monomer <ref name="Li">PMID:34819665</ref>. Next, the kinase domains phosphorylate the [https://en.wikipedia.org/wiki/Tyrosine_phosphorylation tyrosine residues] (Y1278, Y1282, and Y1283 <ref name="Selander-Sunnerhagen">PMID:1527084</ref>) of the opposite monomer using ATP <ref name="Munck">PMID:34646012</ref>. These phosphorylated tyrosine residues recruit signal proteins through phosphorylation. These signal proteins begin a signaling cascade by utilizing various signal pathways including the ERK, JAK, and PI3K pathways. These pathways signal for cell proliferation and survival (ex: begin transcription). The mechanism of tyrosine phosphorylation is a key step in signal transduction and regulation of enzymatic activity. This mechanism is widely used with a variety of proteins and enzymes including being used in insulin signaling. If there is a problem with this mechanism, it can lead to many ill effects as the signaling pathways instigated by this mechanism will be unable to function correctly. | ||
== Applications == | == Applications == | ||
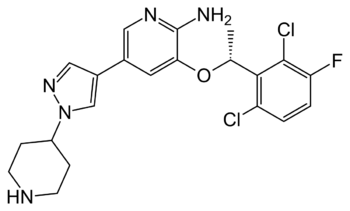
| + | [[Image:Crizotinib_Structure.png|350 px|right|thumb|Figure 5. Structure of the ALK inhibitor, Crizotinib]] | ||
Anaplastic lymphoma kinase's involvement as a proto-oncogene in various types of cancers has made it a target for drug therapies for these types of cancers <ref name="Lewis">PMID:22734674</ref>. These treatments are utilized on the basis that overactivation of the kinase domain of ALK by ATP binding is causing the cells to send out growth signals more rapidly than usual. This overexpression of growth signals allows the cancer cells to grow more rapidly than our normal cells. One therapeutic medication that has been increasingly used with high levels of success is <scene name='90/904309/Crizotinib/1'>Crizotinib</scene>. This medication was approved by the FDA in January of 2021 for the treatment of pediatric/young adult ALK-positive anaplastic large cell lymphoma. ALK-positive cancers are those in which the individual has an oncogenic mutation in their ALK protein sequence that is contributing to the proliferation of the cancer cells. The treatment has an overall response rate of 90%. Crizotinib works by <scene name='90/904309/Crizotinib_bound/1'>binding</scene> to the ATP binding site of the kinase domain <ref name="Sahu">PMID:24455567</ref>. Crizotinib binds preferentially to ATP in this region and therefore can effectively block the binding of ATP <ref name="Sahu" />. With the binding of ATP being blocked, the intercellular signaling cascade cannot begin which works to prevent cancerous cells from growing and spreading . Crizotinib also potently inhibits the RTK, [https://en.wikipedia.org/wiki/C-Met cMET], due to it's kinase domain ATP binding site being very similar to ALK's both sequentially and structurally <ref name="Wang">PMID:25399641</ref>. Other than inhibiting cMET, critotinib is regarded as a highly successful drug as it is very specific to ALK's kinase domain ATP binding site <ref name="Wang">PMID:25399641</ref>. | Anaplastic lymphoma kinase's involvement as a proto-oncogene in various types of cancers has made it a target for drug therapies for these types of cancers <ref name="Lewis">PMID:22734674</ref>. These treatments are utilized on the basis that overactivation of the kinase domain of ALK by ATP binding is causing the cells to send out growth signals more rapidly than usual. This overexpression of growth signals allows the cancer cells to grow more rapidly than our normal cells. One therapeutic medication that has been increasingly used with high levels of success is <scene name='90/904309/Crizotinib/1'>Crizotinib</scene>. This medication was approved by the FDA in January of 2021 for the treatment of pediatric/young adult ALK-positive anaplastic large cell lymphoma. ALK-positive cancers are those in which the individual has an oncogenic mutation in their ALK protein sequence that is contributing to the proliferation of the cancer cells. The treatment has an overall response rate of 90%. Crizotinib works by <scene name='90/904309/Crizotinib_bound/1'>binding</scene> to the ATP binding site of the kinase domain <ref name="Sahu">PMID:24455567</ref>. Crizotinib binds preferentially to ATP in this region and therefore can effectively block the binding of ATP <ref name="Sahu" />. With the binding of ATP being blocked, the intercellular signaling cascade cannot begin which works to prevent cancerous cells from growing and spreading . Crizotinib also potently inhibits the RTK, [https://en.wikipedia.org/wiki/C-Met cMET], due to it's kinase domain ATP binding site being very similar to ALK's both sequentially and structurally <ref name="Wang">PMID:25399641</ref>. Other than inhibiting cMET, critotinib is regarded as a highly successful drug as it is very specific to ALK's kinase domain ATP binding site <ref name="Wang">PMID:25399641</ref>. | ||
Revision as of 17:02, 14 April 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Reshetnyak AV, Rossi P, Myasnikov AG, Sowaileh M, Mohanty J, Nourse A, Miller DJ, Lax I, Schlessinger J, Kalodimos CG. Mechanism for the activation of the anaplastic lymphoma kinase receptor. Nature. 2021 Dec;600(7887):153-157. doi: 10.1038/s41586-021-04140-8. Epub 2021, Nov 24. PMID:34819673 doi:http://dx.doi.org/10.1038/s41586-021-04140-8
- ↑ Palmer RH, Vernersson E, Grabbe C, Hallberg B. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J. 2009 May 27;420(3):345-61. doi: 10.1042/BJ20090387. PMID:19459784 doi:http://dx.doi.org/10.1042/BJ20090387
- ↑ Li T, Stayrook SE, Tsutsui Y, Zhang J, Wang Y, Li H, Proffitt A, Krimmer SG, Ahmed M, Belliveau O, Walker IX, Mudumbi KC, Suzuki Y, Lax I, Alvarado D, Lemmon MA, Schlessinger J, Klein DE. Structural basis for ligand reception by anaplastic lymphoma kinase. Nature. 2021 Dec;600(7887):148-152. doi: 10.1038/s41586-021-04141-7. Epub 2021, Nov 24. PMID:34819665 doi:http://dx.doi.org/10.1038/s41586-021-04141-7
- ↑ Huang H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int J Mol Sci. 2018 Nov 2;19(11). pii: ijms19113448. doi: 10.3390/ijms19113448. PMID:30400214 doi:http://dx.doi.org/10.3390/ijms19113448
- ↑ Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer. 2013 Oct;13(10):685-700. doi: 10.1038/nrc3580. PMID:24060861 doi:http://dx.doi.org/10.1038/nrc3580
- ↑ 6.0 6.1 Selander-Sunnerhagen M, Ullner M, Persson E, Teleman O, Stenflo J, Drakenberg T. How an epidermal growth factor (EGF)-like domain binds calcium. High resolution NMR structure of the calcium form of the NH2-terminal EGF-like domain in coagulation factor X. J Biol Chem. 1992 Sep 25;267(27):19642-9. PMID:1527084
- ↑ 7.0 7.1 Li T, Stayrook SE, Tsutsui Y, Zhang J, Wang Y, Li H, Proffitt A, Krimmer SG, Ahmed M, Belliveau O, Walker IX, Mudumbi KC, Suzuki Y, Lax I, Alvarado D, Lemmon MA, Schlessinger J, Klein DE. Structural basis for ligand reception by anaplastic lymphoma kinase. Nature. 2021 Dec;600(7887):148-152. doi: 10.1038/s41586-021-04141-7. Epub 2021, Nov 24. PMID:34819665 doi:http://dx.doi.org/10.1038/s41586-021-04141-7
- ↑ 8.0 8.1 De Munck S, Provost M, Kurikawa M, Omori I, Mukohyama J, Felix J, Bloch Y, Abdel-Wahab O, Bazan JF, Yoshimi A, Savvides SN. Structural basis of cytokine-mediated activation of ALK family receptors. Nature. 2021 Oct 13. pii: 10.1038/s41586-021-03959-5. doi:, 10.1038/s41586-021-03959-5. PMID:34646012 doi:http://dx.doi.org/10.1038/s41586-021-03959-5
- ↑ Lewis RT, Bode CM, Choquette D, Potashman M, Romero K, Stellwagen JC, Teffera Y, Moore E, Whittington DA, Chen H, Epstein LF, Emkey R, Andrews PS, Yu V, Saffran DC, Xu M, Drew AE, Merkel P, Szilvassy S, Brake RL. The discovery and optimization of a novel class of potent, selective and orally bioavailable Anaplastic Lymphoma Kinase (ALK) Inhibitors with potential utility for the treatment of cancer. J Med Chem. 2012 Jun 26. PMID:22734674 doi:10.1021/jm3005866
- ↑ 10.0 10.1 Sahu A, Prabhash K, Noronha V, Joshi A, Desai S. Crizotinib: A comprehensive review. South Asian J Cancer. 2013 Apr;2(2):91-7. doi: 10.4103/2278-330X.110506. PMID:24455567 doi:http://dx.doi.org/10.4103/2278-330X.110506
- ↑ 11.0 11.1 Wang Q, Zorn JA, Kuriyan J. A structural atlas of kinases inhibited by clinically approved drugs. Methods Enzymol. 2014;548:23-67. doi: 10.1016/B978-0-12-397918-6.00002-1. PMID:25399641 doi:http://dx.doi.org/10.1016/B978-0-12-397918-6.00002-1
PDB Files Used
Student Contributors
- Kaylin Todor
- Rebekah White