We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Neurofibromin
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
[[Image:Nfdomains2.png|800 px|thumb|Figure 1. Domains of Neurofibromin.]] | [[Image:Nfdomains2.png|800 px|thumb|Figure 1. Domains of Neurofibromin.]] | ||
==== GRD domain ==== | ==== GRD domain ==== | ||
| - | The Gap-related domain, or GRD, is the catalytic domain of neurofibromin. This domain also contains a tubulin-binding domain. Its main catalytic mechanism is the hydrolysis of GTP-bound Ras into GDP-bound Ras, which converts Ras from its active form into its inactive form. The GRD provides an arginine residue, known as the arginine finger, to Ras. | + | The Gap-related domain, or GRD, is the catalytic domain of neurofibromin. This domain also contains a tubulin-binding domain. Its main catalytic mechanism is the hydrolysis of GTP-bound Ras into GDP-bound Ras, which converts Ras from its active form into its inactive form. The GRD provides an arginine residue, known as the arginine finger, to Ras. Arg1276 is |
==== SEC-PH ==== | ==== SEC-PH ==== | ||
| - | The Sec-PH domain is the lipid-binding domain of neurofibromin. In the <scene name='90/904326/Sec14ph_and_grd_closed/2'>closed conformation</scene> of neurofibromin, the hydrophobic core is blocked by the Gap-related domain. The <scene name='90/904326/Sec15ph_and_grd_open/2'>open conformation</scene> allows the hydrophobic core in the Sec cavity to be accessible and exposed. | + | The Sec-PH domain is the lipid-binding domain of neurofibromin. In the <scene name='90/904326/Sec14ph_and_grd_closed/2'>closed conformation</scene> of neurofibromin, the hydrophobic core is blocked by the Gap-related domain. The <scene name='90/904326/Sec15ph_and_grd_open/2'>open conformation</scene> allows the hydrophobic core in the Sec cavity to be accessible and exposed. |
==== CSRD and CTD ==== | ==== CSRD and CTD ==== | ||
The Cysteine-Serine-rich domain (CSRD) and C-terminal domain (CTD) contain phosphorylation sites. The CSRD is able to be phosphorylated by protein kinases A and C. Phosphorylation by protein kinase C is a positive regulator of neurofibromin activity. The CTD is phosphorylated primarily by protein kinase C. This domain is a negative regulator of neurofibromin activity if particular residues are phosphorylated. It also plays an important role in tubulin binding, as it helps in the transition from metaphase to anaphase. CTD contains a nuclear localization signal as well. | The Cysteine-Serine-rich domain (CSRD) and C-terminal domain (CTD) contain phosphorylation sites. The CSRD is able to be phosphorylated by protein kinases A and C. Phosphorylation by protein kinase C is a positive regulator of neurofibromin activity. The CTD is phosphorylated primarily by protein kinase C. This domain is a negative regulator of neurofibromin activity if particular residues are phosphorylated. It also plays an important role in tubulin binding, as it helps in the transition from metaphase to anaphase. CTD contains a nuclear localization signal as well. | ||
| Line 17: | Line 17: | ||
=====Closed Conformation===== | =====Closed Conformation===== | ||
The closed state of neurofibromin has both protomers in a closed conformation, which inhibits the binding of Ras to the GRD of neurofibromin due to the HEAT/ARM blocking the GRD. A metal binding site between the N-HEAT/ARM domain and the GRD-Sec14-PH linker stabilize the closed conformation. This site is coordinated by three residues, C1032, H1558, and H1576, and a water molecule. This binding site is preferential for zinc. | The closed state of neurofibromin has both protomers in a closed conformation, which inhibits the binding of Ras to the GRD of neurofibromin due to the HEAT/ARM blocking the GRD. A metal binding site between the N-HEAT/ARM domain and the GRD-Sec14-PH linker stabilize the closed conformation. This site is coordinated by three residues, C1032, H1558, and H1576, and a water molecule. This binding site is preferential for zinc. | ||
| + | ======Zinc Stabilization====== | ||
| + | Zinc has been found to stabilize the closed conformation of neurofibromin. ....... | ||
=====Open Conformation===== | =====Open Conformation===== | ||
The open state of neurofibromin has one protomer in a open conformation and the other in a closed conformation. The protomer in the open conformation allows for the binding of Ras because of reorientation of the GRD and Sec14-PH domains. In the open conformation, the metal binding site found in the closed conformation is lost due to separation of the N-HEAT/ARM and the cysteine residue from the histidine residues founds in the GRD-Sec14-PH linker. | The open state of neurofibromin has one protomer in a open conformation and the other in a closed conformation. The protomer in the open conformation allows for the binding of Ras because of reorientation of the GRD and Sec14-PH domains. In the open conformation, the metal binding site found in the closed conformation is lost due to separation of the N-HEAT/ARM and the cysteine residue from the histidine residues founds in the GRD-Sec14-PH linker. | ||
=====Transition Between Open and Closed Conformation===== | =====Transition Between Open and Closed Conformation===== | ||
| - | + | In the transition from the closed state to the open state, several of the domains of neurofibromin rotate to make the binding site of neurofibromin more accessible . This rotation is able to occur due to the rotation of three connective linkers, L1, L2, and L3. L1 is located between an alpha helix 48 in N-HEAT and an alpha helix 49 in GRD. G1190 is a potential hinge point when L1 rotates and pushes the alpha helixes outwards to move the Gap-related domain. L3 is located between the Sec14-PH domain and the C-HEAT/ARM and aids in the movement of the Sec14-PH domain. Without this rotation, the membrane binding sites are occluded and inaccessible. The proximity of L1 and L3 has to be close to facilitate the rotation of the domains. | |
| - | + | ||
| - | + | ||
====SPRED-1 Protein==== | ====SPRED-1 Protein==== | ||
The SPRED-1 protein <scene name='90/904325/Nf1_ras_spred1/1'>localizes neurofibromin</scene> to the cell membrane in to allow it to bind to the membrane oriented Ras protein<ref name= ''Naschberger''>PMID:34707296</ref>. <scene name='90/904325/Spred_1/1'>SPRED-1</scene> interacts with the N-terminal domain of NF to guide it to the membrane from the cytosol, where its C terminal domain will determine its target <ref name= ''Dunzendorfer-Matt''>PMID:27313208</ref>. | The SPRED-1 protein <scene name='90/904325/Nf1_ras_spred1/1'>localizes neurofibromin</scene> to the cell membrane in to allow it to bind to the membrane oriented Ras protein<ref name= ''Naschberger''>PMID:34707296</ref>. <scene name='90/904325/Spred_1/1'>SPRED-1</scene> interacts with the N-terminal domain of NF to guide it to the membrane from the cytosol, where its C terminal domain will determine its target <ref name= ''Dunzendorfer-Matt''>PMID:27313208</ref>. | ||
Revision as of 18:19, 14 April 2022
| |||||||||||
References
- ↑ Bergoug M, Doudeau M, Godin F, Mosrin C, Vallee B, Benedetti H. Neurofibromin Structure, Functions and Regulation. Cells. 2020 Oct 27;9(11). pii: cells9112365. doi: 10.3390/cells9112365. PMID:33121128 doi:http://dx.doi.org/10.3390/cells9112365
- ↑ Naschberger A, Baradaran R, Rupp B, Carroni M. The structure of neurofibromin isoform 2 reveals different functional states. Nature. 2021 Nov;599(7884):315-319. doi: 10.1038/s41586-021-04024-x. Epub 2021, Oct 27. PMID:34707296 doi:http://dx.doi.org/10.1038/s41586-021-04024-x
- ↑ Trovo-Marqui AB, Tajara EH. Neurofibromin: a general outlook. Clin Genet. 2006 Jul;70(1):1-13. doi: 10.1111/j.1399-0004.2006.00639.x. PMID:16813595 doi:http://dx.doi.org/10.1111/j.1399-0004.2006.00639.x
- ↑ Hall BE, Bar-Sagi D, Nassar N. The structural basis for the transition from Ras-GTP to Ras-GDP. Proc Natl Acad Sci U S A. 2002 Sep 17;99(19):12138-42. Epub 2002 Sep 4. PMID:12213964 doi:http://dx.doi.org/10.1073/pnas.192453199
- ↑ Cimino PJ, Gutmann DH. Neurofibromatosis type 1. Handb Clin Neurol. 2018;148:799-811. doi: 10.1016/B978-0-444-64076-5.00051-X. PMID:29478615 doi:http://dx.doi.org/10.1016/B978-0-444-64076-5.00051-X
- ↑ Bergoug M, Doudeau M, Godin F, Mosrin C, Vallee B, Benedetti H. Neurofibromin Structure, Functions and Regulation. Cells. 2020 Oct 27;9(11). pii: cells9112365. doi: 10.3390/cells9112365. PMID:33121128 doi:http://dx.doi.org/10.3390/cells9112365
- ↑ Naschberger A, Baradaran R, Rupp B, Carroni M. The structure of neurofibromin isoform 2 reveals different functional states. Nature. 2021 Nov;599(7884):315-319. doi: 10.1038/s41586-021-04024-x. Epub 2021, Oct 27. PMID:34707296 doi:http://dx.doi.org/10.1038/s41586-021-04024-x
- ↑ Dunzendorfer-Matt T, Mercado EL, Maly K, McCormick F, Scheffzek K. The neurofibromin recruitment factor Spred1 binds to the GAP related domain without affecting Ras inactivation. Proc Natl Acad Sci U S A. 2016 Jul 5;113(27):7497-502. doi:, 10.1073/pnas.1607298113. Epub 2016 Jun 16. PMID:27313208 doi:http://dx.doi.org/10.1073/pnas.1607298113
- ↑ Frech M, Darden TA, Pedersen LG, Foley CK, Charifson PS, Anderson MW, Wittinghofer A. Role of glutamine-61 in the hydrolysis of GTP by p21H-ras: an experimental and theoretical study. Biochemistry. 1994 Mar 22;33(11):3237-44. doi: 10.1021/bi00177a014. PMID:8136358 doi:http://dx.doi.org/10.1021/bi00177a014
- ↑ Bunda S, Burrell K, Heir P, Zeng L, Alamsahebpour A, Kano Y, Raught B, Zhang ZY, Zadeh G, Ohh M. Inhibition of SHP2-mediated dephosphorylation of Ras suppresses oncogenesis. Nat Commun. 2015 Nov 30;6:8859. doi: 10.1038/ncomms9859. PMID:26617336 doi:http://dx.doi.org/10.1038/ncomms9859
- ↑ Lupton CJ, Bayly-Jones C, D'Andrea L, Huang C, Schittenhelm RB, Venugopal H, Whisstock JC, Halls ML, Ellisdon AM. The cryo-EM structure of the human neurofibromin dimer reveals the molecular basis for neurofibromatosis type 1. Nat Struct Mol Biol. 2021 Dec;28(12):982-988. doi: 10.1038/s41594-021-00687-2., Epub 2021 Dec 9. PMID:34887559 doi:http://dx.doi.org/10.1038/s41594-021-00687-2
- ↑ Abramowicz A, Gos M. Neurofibromin in neurofibromatosis type 1 - mutations in NF1gene as a cause of disease. Dev Period Med. 2014 Jul-Sep;18(3):297-306. PMID:25182393
- ↑ Cimino PJ, Gutmann DH. Neurofibromatosis type 1. Handb Clin Neurol. 2018;148:799-811. doi: 10.1016/B978-0-444-64076-5.00051-X. PMID:29478615 doi:http://dx.doi.org/10.1016/B978-0-444-64076-5.00051-X
- ↑ Ly KI, Blakeley JO. The Diagnosis and Management of Neurofibromatosis Type 1. Med Clin North Am. 2019 Nov;103(6):1035-1054. doi: 10.1016/j.mcna.2019.07.004. PMID:31582003 doi:http://dx.doi.org/10.1016/j.mcna.2019.07.004
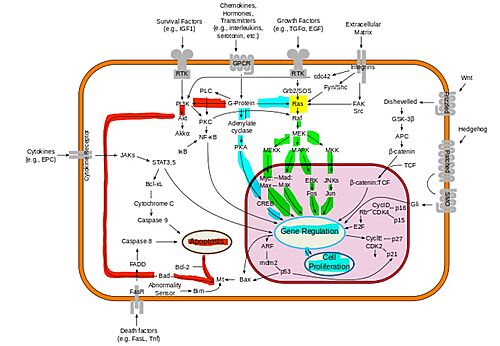
- ↑ McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007 Aug;1773(8):1263-84. doi:, 10.1016/j.bbamcr.2006.10.001. Epub 2006 Oct 7. PMID:17126425 doi:http://dx.doi.org/10.1016/j.bbamcr.2006.10.001
Proteopedia Page Contributors and Editors (what is this?)
Jordyn K. Lenard, Ryan D. Adkins, Michal Harel, OCA, Jaime Prilusky