We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1716
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
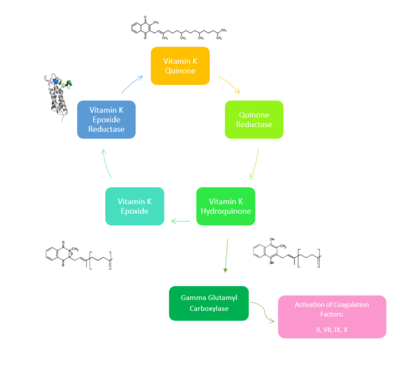
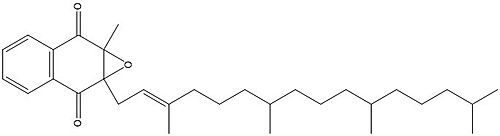
Vitamin K Epoxide Reductase is found and primarily synthesized in the liver. In the liver, the VKOR enzyme is set in the endoplasmic reticulum membrane (Fig.2). The transmembrane helices are located in the Endoplasmic Reticulum Luminal Region, which is the region between the ER Lumen and the Cytosol. The cap region is partially oriented in the ER Lumen. The active site remains within the ER membrane.The Anchor is partially within the ER lumen, and partially embedded in the ER membrane. The anchor is what attaches the cap domain and stabilizes it, which allows the cap domain to cover the active site. Vitamin K Epoxide Reductase is unstable in-vitro. To determine its structure an extra protein superfolder green flourescent protein was appended to the N and C termini of Vitamin K Epoxide. For the visualizing VKOR, this protein has been removed from the structural scenes. After superfolder green flourescent protein was removed from the structural scenes, we further took the structure files and resequenced them to better align with the numbering of the protein. In these files the sequence slightly differs between the organisms used to view Vitamin K Epoxide Reductase. In the human version, HsVKOR, the catalytic cysteines that play an intricate role in the reduction of Vitamin K Epoxide are cysteines 43, 51, 132, and 135. In the pufferfish version of the file, TrVKORL, the cysteines are 52, 55, 141, and 144. | Vitamin K Epoxide Reductase is found and primarily synthesized in the liver. In the liver, the VKOR enzyme is set in the endoplasmic reticulum membrane (Fig.2). The transmembrane helices are located in the Endoplasmic Reticulum Luminal Region, which is the region between the ER Lumen and the Cytosol. The cap region is partially oriented in the ER Lumen. The active site remains within the ER membrane.The Anchor is partially within the ER lumen, and partially embedded in the ER membrane. The anchor is what attaches the cap domain and stabilizes it, which allows the cap domain to cover the active site. Vitamin K Epoxide Reductase is unstable in-vitro. To determine its structure an extra protein superfolder green flourescent protein was appended to the N and C termini of Vitamin K Epoxide. For the visualizing VKOR, this protein has been removed from the structural scenes. After superfolder green flourescent protein was removed from the structural scenes, we further took the structure files and resequenced them to better align with the numbering of the protein. In these files the sequence slightly differs between the organisms used to view Vitamin K Epoxide Reductase. In the human version, HsVKOR, the catalytic cysteines that play an intricate role in the reduction of Vitamin K Epoxide are cysteines 43, 51, 132, and 135. In the pufferfish version of the file, TrVKORL, the cysteines are 52, 55, 141, and 144. | ||
| + | |||
| + | ==Catalytic Cycle== | ||
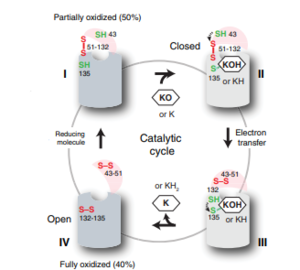
[[Image:VKORcycle.PNG|300px|right|thumb|Figure 2: The catalytic cycle of Vitamin K Epoxide Reductase <ref name=”Shixuan”>PMID:33154105</ref> ]] | [[Image:VKORcycle.PNG|300px|right|thumb|Figure 2: The catalytic cycle of Vitamin K Epoxide Reductase <ref name=”Shixuan”>PMID:33154105</ref> ]] | ||
| Line 26: | Line 28: | ||
| + | ===Step I=== | ||
| + | Step I of reforming Vitamin K Epoxide through the enzyme Vitamin K Reductase (VKOR) begins in a partially oxidized open conformation. In this state, catalytic cysteines 51 and 132 form a disulfide bond. Cysteines 43 and 135 are considered "free" because they are not bound to anything in this state. The <scene name='90/904321/I/2'>central binding pocket</scene> (highlighted in hot pink) is also empty because Vitamin K Epoxide has not bound yet. In order to get to the next step, Vitamin K epoxide will enter through the isoprenyl-chain tunnel.<ref name=”Shixuan”>PMID:33154105</ref> | ||
| + | ===Step II=== | ||
| + | After Vitamin K Epoxide enters through the isoprenyl-chain tunnel, Asn80 on TM2 and Tyr139 on TM4 <scene name='90/904322/Tyr_asn_binding_warfarin/2'>hydrogen bond</scene>. to Vitamin K Epoxide. When Vitamin K Epoxide binds, there is a shift in the bonds between the cap domain, beta hairpin, and anchor. <scene name='90/904321/Vkobound_cys/1'>Cys135</scene> also forms a disulfide bond with the 3' OH group on Vitamin K Epoxide. | ||
| Line 43: | Line 49: | ||
| - | ===Step I=== | ||
| - | Step I of reforming Vitamin K Epoxide through the enzyme Vitamin K Reductase (VKOR) begins in a partially oxidized open conformation. In this state, catalytic cysteines 51 and 132 form a disulfide bond. Cysteines 43 and 135 are considered "free" because they are not bound to anything in this state. The <scene name='90/904321/I/2'>central binding pocket</scene> (highlighted in hot pink) is also empty because Vitamin K Epoxide has not bound yet. In order to get to the next step, Vitamin K epoxide will enter through the isoprenyl-chain tunnel.<ref name=”Shixuan”>PMID:33154105</ref> | ||
| - | ===Step II=== | ||
| - | After Vitamin K Epoxide enters through the isoprenyl-chain tunnel, Asn80 on TM2 and Tyr139 on TM4 <scene name='90/904322/Tyr_asn_binding_warfarin/2'>hydrogen bond</scene>. to Vitamin K Epoxide. When Vitamin K Epoxide binds, there is a shift in the bonds between the cap domain, beta hairpin, and anchor. Cys135 also forms a disulfide bond with the 3' OH group on Vitamin K Epoxide. | ||
Revision as of 15:32, 15 April 2022
Vitamin K Epoxide Reductase
| |||||||||||