We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1716
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
== Introduction == | == Introduction == | ||
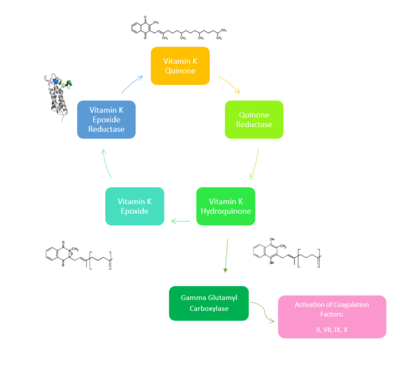
| - | [[Image:NewVitaminKCycle.PNG|400px|right|thumb|'''Figure | + | [[Image:NewVitaminKCycle.PNG|400px|right|thumb|'''Figure 1. Overview of Vitamin K Cycle''': The cycle begins with [https://en.wikipedia.org/wiki/Vitamin_K Vitamin K Quinone]. Vitamin K Quinone is reduced by enzyme Quinone Reductase. This leaves Vitamin K Hydroquinone which can either lead to [https://en.wikipedia.org/wiki/Gamma-glutamyl_carboxylase Gamma Carboxylase]activity that will activate Blood Coagulation Factors II, VII, IX, and X. After this, Vitamin K Epoxide is left over. Vitamin K Epoxide is reduced by the enzyme Vitamin K Epoxide Reductase to reform Vitamin K Quinone. ]] |
<scene name='90/904321/Vitamin_k_epoxide_reductase/1'>Vitamin K Epoxide Reductase</scene> | <scene name='90/904321/Vitamin_k_epoxide_reductase/1'>Vitamin K Epoxide Reductase</scene> | ||
[https://en.wikipedia.org/wiki/Vitamin_K_epoxide_reductase VKOR WIKI](VKOR) is an endoplasmic membrane enzyme that generates the active form of Vitamin K to support blood coagulation<ref name="G. Shen">PMID:33273012</ref>. VKOR homologs are integral membrane thiol oxidoreductases [https://en.wikipedia.org/wiki/Thiol_oxidoreductase Thiol OxidoReductase] due to the function of VKOR being dependent on thiol residues and disulfide bonding. The Vitamin K Cycle and the VKOR enzyme specifically are common drug targets for thromboembolic diseases. This is because, as pictured, the vitamin K cycle is required to activate blood coagulant factors [https://en.wikipedia.org/wiki/Thrombin II], [https://en.wikipedia.org/wiki/Coagulation_factor_VII VII], [https://en.wikipedia.org/wiki/Factor_IX IX], and [https://en.wikipedia.org/wiki/Factor_X#:~:text=Factor%20X%2C%20also%20known%20by,vitamin%20K%20for%20its%20synthesis. X]. Coagulant factor activation promotes blood clotting, which in high amounts can be dangerous and cause thromboembolic diseases such as stroke, deep vein thrombosis, and/or pulmonary embolism. | [https://en.wikipedia.org/wiki/Vitamin_K_epoxide_reductase VKOR WIKI](VKOR) is an endoplasmic membrane enzyme that generates the active form of Vitamin K to support blood coagulation<ref name="G. Shen">PMID:33273012</ref>. VKOR homologs are integral membrane thiol oxidoreductases [https://en.wikipedia.org/wiki/Thiol_oxidoreductase Thiol OxidoReductase] due to the function of VKOR being dependent on thiol residues and disulfide bonding. The Vitamin K Cycle and the VKOR enzyme specifically are common drug targets for thromboembolic diseases. This is because, as pictured, the vitamin K cycle is required to activate blood coagulant factors [https://en.wikipedia.org/wiki/Thrombin II], [https://en.wikipedia.org/wiki/Coagulation_factor_VII VII], [https://en.wikipedia.org/wiki/Factor_IX IX], and [https://en.wikipedia.org/wiki/Factor_X#:~:text=Factor%20X%2C%20also%20known%20by,vitamin%20K%20for%20its%20synthesis. X]. Coagulant factor activation promotes blood clotting, which in high amounts can be dangerous and cause thromboembolic diseases such as stroke, deep vein thrombosis, and/or pulmonary embolism. | ||
| Line 32: | Line 32: | ||
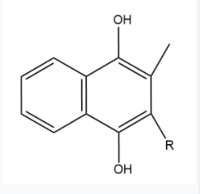
== Vitamin K Epoxide == | == Vitamin K Epoxide == | ||
| - | [[Image:Vitamin K epoxide.jpg|500 px|right|thumb|Figure | + | [[Image:Vitamin K epoxide.jpg|500 px|right|thumb|Figure 2. Vitamin K Epoxide structure]] |
As mentioned above, Vitamin K epoxide is a component of the Vitamin K cycle and required for blood coagulation. In the cycle, VKOR reduces Vitamin K epoxide to Vitamin K Quinone, or the active form of Vitamin K. In this conversion, VKOR donates electrons to Vitamin K epoxide from the S-H of the active pair of cysteines, C132-C135. The mediated cysteine pair, C43-C51, has to be reduced for the transfer of electrons to the substrate to occur. | As mentioned above, Vitamin K epoxide is a component of the Vitamin K cycle and required for blood coagulation. In the cycle, VKOR reduces Vitamin K epoxide to Vitamin K Quinone, or the active form of Vitamin K. In this conversion, VKOR donates electrons to Vitamin K epoxide from the S-H of the active pair of cysteines, C132-C135. The mediated cysteine pair, C43-C51, has to be reduced for the transfer of electrons to the substrate to occur. | ||
| Line 38: | Line 38: | ||
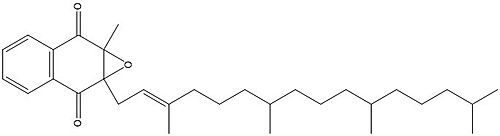
Two other notable structures from the Vitamin K cycle are Vitamin K Quinone (Fig. 5) and Vitamin K Hydroquinone (Fig. 6). Vitamin K Quinone is the product that is released after the reaction with Vitamin K Epoxide and VKOR. (Fig. 2) | Two other notable structures from the Vitamin K cycle are Vitamin K Quinone (Fig. 5) and Vitamin K Hydroquinone (Fig. 6). Vitamin K Quinone is the product that is released after the reaction with Vitamin K Epoxide and VKOR. (Fig. 2) | ||
| - | [[Image:Vitaminkquinone.PNG|200 px|left|thumb|Figure | + | [[Image:Vitaminkquinone.PNG|200 px|left|thumb|Figure 3. Vitamin K Quinone structure]] [[Image:Vitaminkhydroquinone.PNG|200 px|right|thumb|Figure 4. Vitamin K Hydroquinone structure]] |
=== Binding === | === Binding === | ||
| Line 46: | Line 46: | ||
==Catalytic Cycle== | ==Catalytic Cycle== | ||
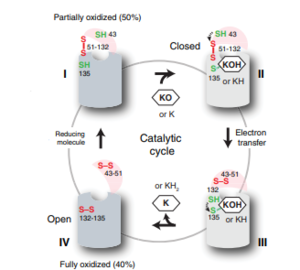
| - | [[Image:VKORcycle.PNG|300px|right|thumb|Figure | + | [[Image:VKORcycle.PNG|300px|right|thumb|'''Figure 5: The catalytic cycle of Vitamin K Epoxide Reductase''' <ref name=”Shixuan”>PMID:33154105</ref> ]] |
| Line 56: | Line 56: | ||
===Step I === | ===Step I === | ||
| - | <scene name='90/904321/I/1'>Step I</scene> of reforming Vitamin K Epoxide through the enzyme Vitamin K Reductase (VKOR) begins in a partially oxidized open conformation. In this state, catalytic cysteines 51 and 132 form a disulfide bond. Cysteines 43 and 135 are considered "free" because they are not bound to anything in this state. The <scene name='90/904321/I/2'>central binding pocket</scene> (highlighted in hot pink) is also empty because Vitamin K Epoxide has not bound yet. In order to get to the next step, Vitamin K epoxide will enter through the isoprenyl-chain tunnel.<ref name=”Shixuan”>PMID:33154105</ref> | + | <scene name='90/904321/I/1'>Step I</scene> of reforming Vitamin K Epoxide (Fig. 3) through the enzyme Vitamin K Reductase (VKOR) begins in a partially oxidized open conformation. In this state, catalytic cysteines 51 and 132 form a disulfide bond. Cysteines 43 and 135 are considered "free" because they are not bound to anything in this state. The <scene name='90/904321/I/2'>central binding pocket</scene> (highlighted in hot pink) is also empty because Vitamin K Epoxide has not bound yet. In order to get to the next step, Vitamin K epoxide will enter through the isoprenyl-chain tunnel.<ref name=”Shixuan”>PMID:33154105</ref> |
===Step II=== | ===Step II=== | ||
| Line 62: | Line 62: | ||
===Step III=== | ===Step III=== | ||
| - | <scene name='90/904321/Iii/1'>Step III</scene> is within this partially <scene name='90/904321/Iii/2'>oxidized state</scene> and free Cys43 forms a bond with Cys 51. Cys51 kicks its electrons to Cys132, and Cys 132 forms a disulfide bond with Cys135. Cys135 then reduces the epoxide ring on Vitamin K Epoxide after donating its electrons. The epoxide ring opens and reforms Vitamin K Quinone. | + | <scene name='90/904321/Iii/1'>Step III</scene> is within this partially <scene name='90/904321/Iii/2'>oxidized state</scene> and free Cys43 forms a bond with Cys 51. Cys51 kicks its electrons to Cys132, and Cys 132 forms a disulfide bond with Cys135. Cys135 then reduces the epoxide ring on Vitamin K Epoxide after donating its electrons. The epoxide ring opens and reforms Vitamin K Quinone (Fig.3). |
===Step IV=== | ===Step IV=== | ||
| Line 70: | Line 70: | ||
== Inhibition of VKOR == | == Inhibition of VKOR == | ||
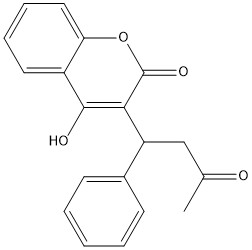
[https://en.wikipedia.org/wiki/Warfarin Warfarin] is the most common [https://en.wikipedia.org/wiki/Vitamin_K_antagonist Vitamin K antagonist (VKA)]. Warfarin is a competitive inhibitor, taking the place of Vitamin K Epoxide (VKO) in the active site of Vitamin K Epoxide Reductase (VKOR). When warfarin binds in the active site, it causes VKOR to go into the closed conformation. | [https://en.wikipedia.org/wiki/Warfarin Warfarin] is the most common [https://en.wikipedia.org/wiki/Vitamin_K_antagonist Vitamin K antagonist (VKA)]. Warfarin is a competitive inhibitor, taking the place of Vitamin K Epoxide (VKO) in the active site of Vitamin K Epoxide Reductase (VKOR). When warfarin binds in the active site, it causes VKOR to go into the closed conformation. | ||
| - | [[Image:warfarin.jpg|400 px|right|thumb|Figure | + | [[Image:warfarin.jpg|400 px|right|thumb|Figure 6. 2-Dimensional structure of Warfarin]] |
=== Binding === | === Binding === | ||
Revision as of 16:16, 15 April 2022
Vitamin K Epoxide Reductase
| |||||||||||