Sandbox Reserved 1705
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
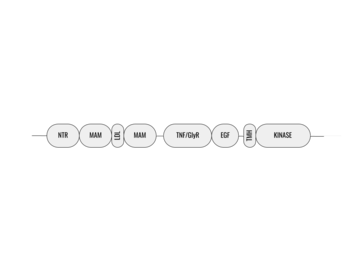
[[Image:ALK_Domain_Outline.png|350 px|right|thumb|Figure 1. Outline of the domains and regions of anaplastic lymphoma kinase]] | [[Image:ALK_Domain_Outline.png|350 px|right|thumb|Figure 1. Outline of the domains and regions of anaplastic lymphoma kinase]] | ||
Anaplastic lymphoma kinase is a [https://en.wikipedia.org/wiki/Receptor_tyrosine_kinase receptor tyrosine kinase] (RTK) that is important in regulating functions within the central nervous system <ref name="Reshetnyak">PMID:34819673</ref>. RTKs are the high-affinity cell surface receptors for many polypeptide growth factors, cytokines, and hormones. ALK activates many pathways including the [https://en.wikipedia.org/wiki/MAPK/ERK_pathway ERK], [https://en.wikipedia.org/wiki/JAK-STAT_signaling_pathway JAK], and [https://en.wikipedia.org/wiki/PI3K/AKT/mTOR_pathway PI3K] pathways, all of which are involved in proliferation, migration, and cell survival. ALK is composed of two identical monomers consisting of seven unique domains and two intermixed regions. One region to note is the glycine-rich region which is highly uncharacteristic in that it has helices composed of only glycine. The preferred ligand for binding is AUG which binds in a dimeric fashion to ALK. When the ligand has bound, there is a conformational change that is covered in more detail in Figure 2 and it's accompanying text. In order to determine the atomic details of human ALK dimerization and activation by AUG, the methods of [https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy cryo-electron microscopy],[https://en.wikipedia.org/wiki/Nuclear_magnetic_resonance_spectroscopy nuclear magnetic resonance], and [https://en.wikipedia.org/wiki/X-ray_crystallography X-ray crystallography] were utilized <ref name="Reshetnyak">PMID:34819673</ref> . Anaplastic lymphoma kinase is a proto-oncogene with mutations associated with various types of cancers, including non-small-cell lung cancer, anaplastic large cell lymphoma, squamous cell carcinoma, and inflammatory myofibroblastic cancer <ref name="Palmer">PMID:19459784</ref>. ALK is a referred to as a proto-oncogene because certain mutations in it's protein sequence are known to have a strong positive association with the development of cancerous cells. | Anaplastic lymphoma kinase is a [https://en.wikipedia.org/wiki/Receptor_tyrosine_kinase receptor tyrosine kinase] (RTK) that is important in regulating functions within the central nervous system <ref name="Reshetnyak">PMID:34819673</ref>. RTKs are the high-affinity cell surface receptors for many polypeptide growth factors, cytokines, and hormones. ALK activates many pathways including the [https://en.wikipedia.org/wiki/MAPK/ERK_pathway ERK], [https://en.wikipedia.org/wiki/JAK-STAT_signaling_pathway JAK], and [https://en.wikipedia.org/wiki/PI3K/AKT/mTOR_pathway PI3K] pathways, all of which are involved in proliferation, migration, and cell survival. ALK is composed of two identical monomers consisting of seven unique domains and two intermixed regions. One region to note is the glycine-rich region which is highly uncharacteristic in that it has helices composed of only glycine. The preferred ligand for binding is AUG which binds in a dimeric fashion to ALK. When the ligand has bound, there is a conformational change that is covered in more detail in Figure 2 and it's accompanying text. In order to determine the atomic details of human ALK dimerization and activation by AUG, the methods of [https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy cryo-electron microscopy],[https://en.wikipedia.org/wiki/Nuclear_magnetic_resonance_spectroscopy nuclear magnetic resonance], and [https://en.wikipedia.org/wiki/X-ray_crystallography X-ray crystallography] were utilized <ref name="Reshetnyak">PMID:34819673</ref> . Anaplastic lymphoma kinase is a proto-oncogene with mutations associated with various types of cancers, including non-small-cell lung cancer, anaplastic large cell lymphoma, squamous cell carcinoma, and inflammatory myofibroblastic cancer <ref name="Palmer">PMID:19459784</ref>. ALK is a referred to as a proto-oncogene because certain mutations in it's protein sequence are known to have a strong positive association with the development of cancerous cells. | ||
| + | |||
| + | |||
== General Structure == | == General Structure == | ||
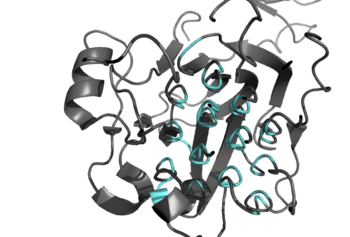
[[Image:Ray_Traced_GlyR_Image.png|350 px|left|thumb|Figure 2: Shown in teal is the Glycine Rich Region of ALK in its helical structure. ]] | [[Image:Ray_Traced_GlyR_Image.png|350 px|left|thumb|Figure 2: Shown in teal is the Glycine Rich Region of ALK in its helical structure. ]] | ||
Anaplastic lymphoma kinase is a <scene name='90/904310/Dimer/5'>homodimer</scene>, each <scene name='90/904310/Monomer_a/3'>monomer</scene> consisting of seven domains and two regions <ref name="Tongqing">PMID:34819665</ref>. These domains and regions are as follows: N-terminal region (NTR), two meprin–A-5 protein–receptor protein tyrosine phosphatase μ domains (MAM), low density lipoprotein receptor class A domain (LDL), <scene name='90/904310/Tnf_highlighted_monomer/5'>tumor necrosis factor receptor-like domain</scene> (TNF), <scene name='90/904310/Glyr_highlighted_monomer/5'>glycine rich region</scene> (GlyR), <scene name='90/904310/Egf_highlighted_monomer/3'>epidermal growth factor receptor-like domain</scene> (EGF), transmembrane α-helix (TMH), kinase domain <ref name="Reshetnyak">PMID:34819673</ref>. The NTR functions as a signal peptide, the structure of which is yet to be determined. Though the biological roles and structures of MAM and LDL have not been determined, they are a very unique component to ALK. ALK is the only RTK that has two MAM domains and a LDL domain. Studies of other MAM domains have suggested that MAM may play a role in cell-cell interactions through homophilic binding <ref name="Huang">PMID: 30400214</ref>. The TNF-like domain assists in mediating mature T-cell receptor induced apoptosis. Both the TNF domain and GlyR region are discontinuous, traversing each other frequently <ref name="Reshetnyak">PMID:34819673</ref>. The GlyR region consists of multiple glycine helices which is a highly unique structure. Though the function of ALK's EGF domain is unknown, we do know that all EGF domains are found in the extracellular region and are thought to be important building blocks for extracellular proteins <ref name="Hallberg">PMID:24060861</ref>. The TMH connects the extracellular and intracellular regions of ALK through the plasma membrane. The kinase domain is in the intracellular region and is phosphorylated at positions <scene name='90/904309/Tyrosines/1'>Y1278, Y1282, and Y1283</scene> through the tyrosine phosphorylation mechanism in order to begin signaling cascades <ref name="Selander-Sunnerhagen">PMID:1527084</ref>. The structures of the N-terminal region, MAM, and LDL have not been determined. Only the TNF, GlyR, and EGF portions of ALK are required for ligand binding. All portions of anaplastic lymphoma kinase are located in the extracellular domain except for the transmembrane α-helix which is in the transmembrane region and the kinase domain that is located in the intracellular region. | Anaplastic lymphoma kinase is a <scene name='90/904310/Dimer/5'>homodimer</scene>, each <scene name='90/904310/Monomer_a/3'>monomer</scene> consisting of seven domains and two regions <ref name="Tongqing">PMID:34819665</ref>. These domains and regions are as follows: N-terminal region (NTR), two meprin–A-5 protein–receptor protein tyrosine phosphatase μ domains (MAM), low density lipoprotein receptor class A domain (LDL), <scene name='90/904310/Tnf_highlighted_monomer/5'>tumor necrosis factor receptor-like domain</scene> (TNF), <scene name='90/904310/Glyr_highlighted_monomer/5'>glycine rich region</scene> (GlyR), <scene name='90/904310/Egf_highlighted_monomer/3'>epidermal growth factor receptor-like domain</scene> (EGF), transmembrane α-helix (TMH), kinase domain <ref name="Reshetnyak">PMID:34819673</ref>. The NTR functions as a signal peptide, the structure of which is yet to be determined. Though the biological roles and structures of MAM and LDL have not been determined, they are a very unique component to ALK. ALK is the only RTK that has two MAM domains and a LDL domain. Studies of other MAM domains have suggested that MAM may play a role in cell-cell interactions through homophilic binding <ref name="Huang">PMID: 30400214</ref>. The TNF-like domain assists in mediating mature T-cell receptor induced apoptosis. Both the TNF domain and GlyR region are discontinuous, traversing each other frequently <ref name="Reshetnyak">PMID:34819673</ref>. The GlyR region consists of multiple glycine helices which is a highly unique structure. Though the function of ALK's EGF domain is unknown, we do know that all EGF domains are found in the extracellular region and are thought to be important building blocks for extracellular proteins <ref name="Hallberg">PMID:24060861</ref>. The TMH connects the extracellular and intracellular regions of ALK through the plasma membrane. The kinase domain is in the intracellular region and is phosphorylated at positions <scene name='90/904309/Tyrosines/1'>Y1278, Y1282, and Y1283</scene> through the tyrosine phosphorylation mechanism in order to begin signaling cascades <ref name="Selander-Sunnerhagen">PMID:1527084</ref>. The structures of the N-terminal region, MAM, and LDL have not been determined. Only the TNF, GlyR, and EGF portions of ALK are required for ligand binding. All portions of anaplastic lymphoma kinase are located in the extracellular domain except for the transmembrane α-helix which is in the transmembrane region and the kinase domain that is located in the intracellular region. | ||
| + | |||
=== Ligand Binding=== | === Ligand Binding=== | ||
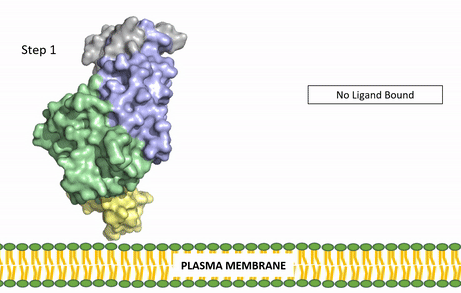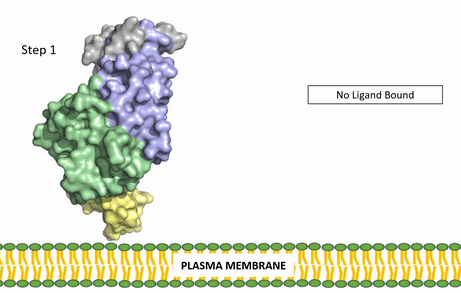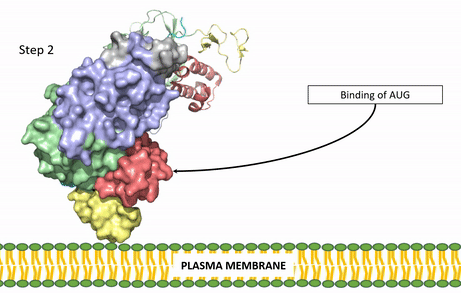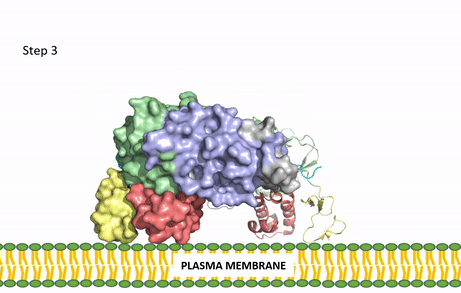
[[Image:ALK Conformational Change Gif.gif|850 px|right|thumb|Figure 3: Gif-image of the conformational change occurring in the extracellular region of Anaplastic Lymphoma Kinase once the AUG ligand has bound to the ligand-binding site. This change is stabilized through contacts of the AUG and the plasma membrane. The video was made using stop motion animation techniques, then converted to gif format using EZgif.]] | [[Image:ALK Conformational Change Gif.gif|850 px|right|thumb|Figure 3: Gif-image of the conformational change occurring in the extracellular region of Anaplastic Lymphoma Kinase once the AUG ligand has bound to the ligand-binding site. This change is stabilized through contacts of the AUG and the plasma membrane. The video was made using stop motion animation techniques, then converted to gif format using EZgif.]] | ||
The ligands recognized by anaplastic lymphoma kinase are FAM150 in a monomeric fashion and <scene name='90/904310/Ligand/2'>AUG</scene> in a dimeric fashion <ref name="Li">PMID:34819665</ref> . Its biologically preferred ligand is AUG, a 128 monomer peptide ligand. FAM150 is a structural ortholog of AUG. The binding of ALK to it's ligand results in homodimerization and a conformational change. Prior to the ligand binding to anaplastic lymphoma kinase, the extracellular domain is oriented vertically and perpendicularly to the plasma membrane (Step 1, Figure 3). Once the ligand is <scene name='90/904310/Dimer_ligand_complex/5'>bound</scene> (Step 2, Figure 3), ALK undergoes a conformational change and folds over so that the positively charged residues on the portion of the protein previously oriented vertically is now interacting with the negatively charged residues on the plasma membrane (Step 3, Figure 3). The residues of ALK and it's ligand interact through the formation of <scene name='90/904310/Dimer-ligand-interface/5'>salt bridges</scene><ref name="Munck">PMID:34646012</ref>. It has been hypothesized that the GlyR region plays a role in the flexibility needed to complete the conformational change. This conformational change via ligand binding induces the auto-activation of the kinase domain, in which the domains use the tyrosine phosphorylation mechanism to phosphorylate tyrosine residues on the opposite monomer. Another well-known RTK is the insulin receptor which is activated using a similar pathway. To learn more about the larger mechanism of RTK activation, click [https://proteopedia.org/wiki/index.php/Insulin_receptor#:~:text=The%20insulin%20receptor%20binds%20the,including%20skeletal%20muscle%20and%20adipose here]. | The ligands recognized by anaplastic lymphoma kinase are FAM150 in a monomeric fashion and <scene name='90/904310/Ligand/2'>AUG</scene> in a dimeric fashion <ref name="Li">PMID:34819665</ref> . Its biologically preferred ligand is AUG, a 128 monomer peptide ligand. FAM150 is a structural ortholog of AUG. The binding of ALK to it's ligand results in homodimerization and a conformational change. Prior to the ligand binding to anaplastic lymphoma kinase, the extracellular domain is oriented vertically and perpendicularly to the plasma membrane (Step 1, Figure 3). Once the ligand is <scene name='90/904310/Dimer_ligand_complex/5'>bound</scene> (Step 2, Figure 3), ALK undergoes a conformational change and folds over so that the positively charged residues on the portion of the protein previously oriented vertically is now interacting with the negatively charged residues on the plasma membrane (Step 3, Figure 3). The residues of ALK and it's ligand interact through the formation of <scene name='90/904310/Dimer-ligand-interface/5'>salt bridges</scene><ref name="Munck">PMID:34646012</ref>. It has been hypothesized that the GlyR region plays a role in the flexibility needed to complete the conformational change. This conformational change via ligand binding induces the auto-activation of the kinase domain, in which the domains use the tyrosine phosphorylation mechanism to phosphorylate tyrosine residues on the opposite monomer. Another well-known RTK is the insulin receptor which is activated using a similar pathway. To learn more about the larger mechanism of RTK activation, click [https://proteopedia.org/wiki/index.php/Insulin_receptor#:~:text=The%20insulin%20receptor%20binds%20the,including%20skeletal%20muscle%20and%20adipose here]. | ||
| + | |||
| + | |||
| + | |||
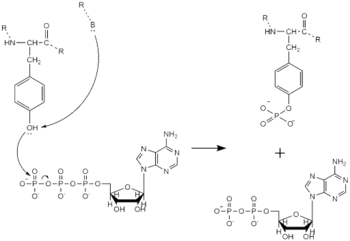
==== Tyrosine Phosphorylation Mechanism ==== | ==== Tyrosine Phosphorylation Mechanism ==== | ||
[[Image:Tyrosine_Mechanism_Picture.png|350 px|left|thumb|Figure 4. Tyrosine phosphorylation mechanism]] | [[Image:Tyrosine_Mechanism_Picture.png|350 px|left|thumb|Figure 4. Tyrosine phosphorylation mechanism]] | ||
| + | |||
| + | |||
| + | |||
Dimerization of anaplastic lymphoma kinase activates the <scene name='90/904309/Kinase_domain/2'>kinase domain</scene> of each monomer <ref name="Li">PMID:34819665</ref>. Next, the kinase domains phosphorylate the [https://en.wikipedia.org/wiki/Tyrosine_phosphorylation tyrosine residues] (Y1278, Y1282, and Y1283 <ref name="Selander-Sunnerhagen">PMID:1527084</ref>) of the opposite monomer using ATP <ref name="Munck">PMID:34646012</ref>. These phosphorylated tyrosine residues recruit signal proteins through phosphorylation. These signal proteins begin a signaling cascade by utilizing various signal pathways including the ERK, JAK, and PI3K pathways. These pathways signal for cell proliferation and survival (ex: begin transcription). The mechanism of tyrosine phosphorylation is a key step in signal transduction and regulation of enzymatic activity. This mechanism is widely used with a variety of proteins and enzymes including being used in insulin signaling. If there is a problem with this mechanism, it can lead to many ill effects as the signaling pathways instigated by this mechanism will be unable to function correctly. | Dimerization of anaplastic lymphoma kinase activates the <scene name='90/904309/Kinase_domain/2'>kinase domain</scene> of each monomer <ref name="Li">PMID:34819665</ref>. Next, the kinase domains phosphorylate the [https://en.wikipedia.org/wiki/Tyrosine_phosphorylation tyrosine residues] (Y1278, Y1282, and Y1283 <ref name="Selander-Sunnerhagen">PMID:1527084</ref>) of the opposite monomer using ATP <ref name="Munck">PMID:34646012</ref>. These phosphorylated tyrosine residues recruit signal proteins through phosphorylation. These signal proteins begin a signaling cascade by utilizing various signal pathways including the ERK, JAK, and PI3K pathways. These pathways signal for cell proliferation and survival (ex: begin transcription). The mechanism of tyrosine phosphorylation is a key step in signal transduction and regulation of enzymatic activity. This mechanism is widely used with a variety of proteins and enzymes including being used in insulin signaling. If there is a problem with this mechanism, it can lead to many ill effects as the signaling pathways instigated by this mechanism will be unable to function correctly. | ||
| + | |||
| + | |||
| + | |||
| + | |||
Revision as of 19:37, 15 April 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Reshetnyak AV, Rossi P, Myasnikov AG, Sowaileh M, Mohanty J, Nourse A, Miller DJ, Lax I, Schlessinger J, Kalodimos CG. Mechanism for the activation of the anaplastic lymphoma kinase receptor. Nature. 2021 Dec;600(7887):153-157. doi: 10.1038/s41586-021-04140-8. Epub 2021, Nov 24. PMID:34819673 doi:http://dx.doi.org/10.1038/s41586-021-04140-8
- ↑ Palmer RH, Vernersson E, Grabbe C, Hallberg B. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J. 2009 May 27;420(3):345-61. doi: 10.1042/BJ20090387. PMID:19459784 doi:http://dx.doi.org/10.1042/BJ20090387
- ↑ Li T, Stayrook SE, Tsutsui Y, Zhang J, Wang Y, Li H, Proffitt A, Krimmer SG, Ahmed M, Belliveau O, Walker IX, Mudumbi KC, Suzuki Y, Lax I, Alvarado D, Lemmon MA, Schlessinger J, Klein DE. Structural basis for ligand reception by anaplastic lymphoma kinase. Nature. 2021 Dec;600(7887):148-152. doi: 10.1038/s41586-021-04141-7. Epub 2021, Nov 24. PMID:34819665 doi:http://dx.doi.org/10.1038/s41586-021-04141-7
- ↑ Huang H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int J Mol Sci. 2018 Nov 2;19(11). pii: ijms19113448. doi: 10.3390/ijms19113448. PMID:30400214 doi:http://dx.doi.org/10.3390/ijms19113448
- ↑ Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer. 2013 Oct;13(10):685-700. doi: 10.1038/nrc3580. PMID:24060861 doi:http://dx.doi.org/10.1038/nrc3580
- ↑ 6.0 6.1 Selander-Sunnerhagen M, Ullner M, Persson E, Teleman O, Stenflo J, Drakenberg T. How an epidermal growth factor (EGF)-like domain binds calcium. High resolution NMR structure of the calcium form of the NH2-terminal EGF-like domain in coagulation factor X. J Biol Chem. 1992 Sep 25;267(27):19642-9. PMID:1527084
- ↑ 7.0 7.1 Li T, Stayrook SE, Tsutsui Y, Zhang J, Wang Y, Li H, Proffitt A, Krimmer SG, Ahmed M, Belliveau O, Walker IX, Mudumbi KC, Suzuki Y, Lax I, Alvarado D, Lemmon MA, Schlessinger J, Klein DE. Structural basis for ligand reception by anaplastic lymphoma kinase. Nature. 2021 Dec;600(7887):148-152. doi: 10.1038/s41586-021-04141-7. Epub 2021, Nov 24. PMID:34819665 doi:http://dx.doi.org/10.1038/s41586-021-04141-7
- ↑ 8.0 8.1 De Munck S, Provost M, Kurikawa M, Omori I, Mukohyama J, Felix J, Bloch Y, Abdel-Wahab O, Bazan JF, Yoshimi A, Savvides SN. Structural basis of cytokine-mediated activation of ALK family receptors. Nature. 2021 Oct 13. pii: 10.1038/s41586-021-03959-5. doi:, 10.1038/s41586-021-03959-5. PMID:34646012 doi:http://dx.doi.org/10.1038/s41586-021-03959-5
- ↑ Lewis RT, Bode CM, Choquette D, Potashman M, Romero K, Stellwagen JC, Teffera Y, Moore E, Whittington DA, Chen H, Epstein LF, Emkey R, Andrews PS, Yu V, Saffran DC, Xu M, Drew AE, Merkel P, Szilvassy S, Brake RL. The discovery and optimization of a novel class of potent, selective and orally bioavailable Anaplastic Lymphoma Kinase (ALK) Inhibitors with potential utility for the treatment of cancer. J Med Chem. 2012 Jun 26. PMID:22734674 doi:10.1021/jm3005866
- ↑ 10.0 10.1 Sahu A, Prabhash K, Noronha V, Joshi A, Desai S. Crizotinib: A comprehensive review. South Asian J Cancer. 2013 Apr;2(2):91-7. doi: 10.4103/2278-330X.110506. PMID:24455567 doi:http://dx.doi.org/10.4103/2278-330X.110506
- ↑ 11.0 11.1 Wang Q, Zorn JA, Kuriyan J. A structural atlas of kinases inhibited by clinically approved drugs. Methods Enzymol. 2014;548:23-67. doi: 10.1016/B978-0-12-397918-6.00002-1. PMID:25399641 doi:http://dx.doi.org/10.1016/B978-0-12-397918-6.00002-1
PDB Files Used
Student Contributors
- Kaylin Todor
- Rebekah White