Introduction
Biological Role
(VKOR) is a reducing enzyme composed of 4-helices that spans the endoplasmic reticulum as a transmembrane protein
[1]. Its enzymatic role is reducing (KO) to Vitamin K Hydroquinone (KH2)
[2] (Figure 1). The mechanism first occurs through the binding KO and using two cysteine residues to reduce KO into
Vitamin K. Then, a second pair of cysteine residues will reduce Vitamin K into the final product, KH2 (Figure 1). One of VKORs primary roles is to assist in blood coagulation through this KH2 regeneration mechanism.
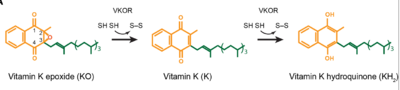
Figure 1. Mechanism of KO reduction into KH2.
With Vitamin K as a cofactor, the
γ-carboxylase enzyme will enact post-translational modification on KH2, oxidizing it back to KO. The oxidation of KH2 by γ-carboxylase is coupled with the carboxylation of a glutamate residue to form γ-carboxyglutamate. The coupling of this oxidation and carboxylation will activate several clotting factors in the coagulation cascade.
Author's Notes
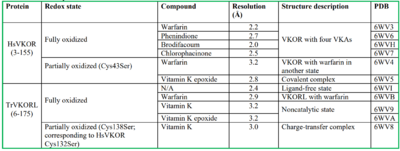
Structural characterization of VKOR has been difficult due to its in vitro instability. Recently, a series of atomic structures have been determined utilizing anticoagulant stabilization and VKOR-like homologs. Crystal structures of VKOR were captured with a bound substrate (KO) or vitamin K antagonist (VKA)[3]. VKA substrates utilized were anticoagulants, namely Warfarin, Brodifacoum, Phenindione, and Chlorophacinone. Second, VKOR-like homologs were utilized to aid in structure classification. Homologs refer to specific cysteine residues that have been mutated to serine to facilitate capturing a stable conformation state. Homologs were mainly isolated from human VKOR with some isolated from the pufferfish Takifugu rubripes. Furthermore, all of the structures used have been processed to remove a beta barrel at the south end of VKOR that served no purpose in function of the enzyme. This also allowed for the residue numbering to be reassigned and more closely replicate the human VKOR.
Structural Highlights
VKOR has many key structural components that allow it to maintain proper functionality and catalytic abilities. The main part of the enzyme that contains the active site is a binding pocket where main catalytic activity occurs. The VKOR binding pocket provides specific substrate binding via highly conserved residues that recognize the target substrates. The pocket works in conjunction with the cap domain. The cap domain is a helical component of VKOR that facilitates conformational transitions from the to the once a substrate binds. Interactions between the cap domain, binding pocket, and the bound protein are critical to achieve full activation of Vitamin K. Another necessary part of the structure is the anchor. The anchor serves as a way to hold VKOR in the proper orientation within the cell membrane such that all enzymatic components are in the correct proximity for substrate binding and catalysis. Vital to the VKOR structure and these components are two disulfide bridges. The first appears slightly above the binding pocket between C132 and C135. The second occurs within the cap domain between C43 and C51. These cysteines are catalytic residues that also aid in the transition of VKOR from the open conformation to the closed conformation and the reduction of KO.
Active Site
Within the four transmembrane helices lies the . The binding pocket is comprised of a containing , N80 and Y139, that interact with substrates. The hydrophobic pocket provides specificity to the region while the hydrophilic residues hydrogen bond to the substrate, providing recognition and increasing specificity. The above the binding pocket provides stabilization when a substrate is bound. This bridge provides increased stability for the binding site as it interacts with and binds substrates or inhibitors. The hydrophilic residues provide when interacting with substrates for specificity and recognition. Upon binding, VKOR will transition into the closed conformation allowing the catalytic mechanism to commence.
Cap Domain
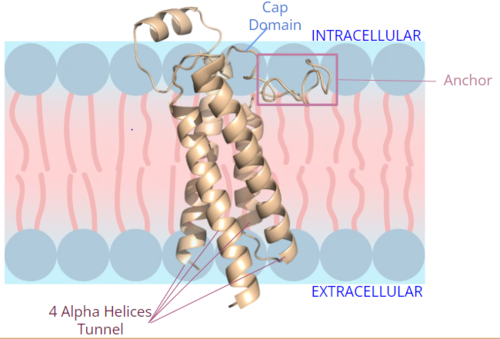
Figure 2. Orientation and interactions of the VKOR components cap domain, anchor domain, and helical tunnel within the cell membrane.
A key part of VKOR is the function of the , which is located right above the helices of VKOR towards the intracellular part of the membrane. The cap has a helical shape and is located in close proximity to two other domains: the Anchor domain and beta hairpin. This combination of domains help to maintain the proper orientation in the membrane. The cap domain assists with activating Vitamin K as it induces the structural change of VKOR from the open conformation to the closed conformation upon substrate binding. Cap rearrangement and transition to the closed conformation initiates a domino effect through the catalytic mechanism. The cap domain has critical interactions that stabilize the closed conformation including a between C43 and C51, and polar interactions from D44. These interactions are broken up by reactive cysteines to induce different conformations and help facilitate this transition from the open conformation to the closed conformation during the activation of Vitamin K.
Anchor
The is a key part of the VKOR structure and function that protrudes from the side of VKOR with the primary role of stabilizing the enzyme within the membrane. It sits on top of the membrane surface, as shown in figure 2, such that anchor residues can interact with the cell membrane to maintain proper proximity for VKOR activity. To accomplish this, hydrophilic residues are positioned to interact with the outer hydrophilic leaflet of the bilipid membrane, while the hydrophobic residues on the anchor have strong interactions with the inner hydrophobic leaflet of the bilipid membrane. These allow for VKOR to remain in the proper membrane arrangement and proximity for Vitamin K to bind and be activated via the cap domain and active site. The anchor also serves a role in connecting the cap domain to the rest of the membrane so that it stabilizes its covering of the central binding pocket to keep the substrate within the active site during its catalytic activation. These membrane interactions allow for VKOR to stabilize in the membrane for proper activation of Vitamin K and catalytic function of the enzyme.
Catalytic Mechanism of VKOR
Brief Overview
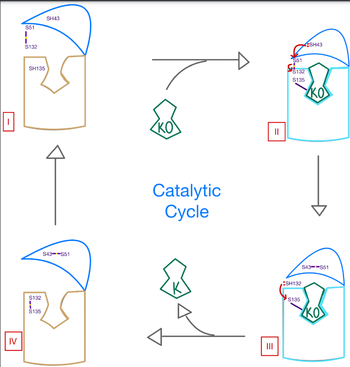
The overall mechanism works to convert Vitamin K epoxide to an activated form of Vitamin K hydroquinone, as noted in Figure 1. The substrate will bind VKOR at the binding pocket in the and induce the . Transition from open to closed conformation occurs with the oxidation of the C43-C51 disulfide bridge. Here, VKOR will utilize the second pair of , C132 and C135, to reduce KO into Vitamin K and Vitamin K into KH2. KH2 will be released from the binding fully activated and ready for use in the body. VKOR will reset, returning to the open conformation again, prepared for another substrate to bind.
Enzymatic Mechanism
The catalytic mechanism of VKOR is highly regulated and use to activate Vitamin K necessary for blood coagulation. Figure 3 highlights these reactions that allow the substrate to be catalyzed to its active form through a series of 4 stages. The enzyme begins in in the open conformation with the cap domain open to allow substrate binding. Once a substrate binds, the cap domain transitions to the closed conformation when the C51-C132 disulfide bridge is attacked by reactive C43 located within the cap domain. This reaction forms a new disulfide bridge between C43 and C51 that pulls the cap domain over the binding pocket with the substrate bound to stabilize the closed conformation of VKOR. VKOR is now in . Free cysteines are now available that provide strong stabilization of the closed conformation through interactions with the cap domain and the bound substrate. This puts the enzyme in , where a free C135 is purposed to interact with the substrate within the binding pocket to stabilize it during activation. The catalytic free C132 located between the cap domain and helical tunnel is very reactive and will attack this C135 to break that interaction with the substrate and release the activated Vitamin K product into the blood stream to promote coagulation. Two very stable disulfide bridges between C43-C41 and C132-C135 are now present and VKOR is unbound, so the enzyme is in its final, unreactive . VKOR must undergo conformational changes to return to Stage 1 and reactivate its catalytic cysteines so that another molecule of Vitamin K can bind and be activated.
Disease and Treatment
Afflictions
Since activated Vitamin K plays a crucial role in blood coagulation, defects in the function and enzymatic activity of VKOR may detrimentally effect on Vitamin K's ability to promote blood clotting. Mutations in VKOR also increase susceptibility to vascular diseases, such as a stroke [1]. Vitamin K is also important in maintaining bone health with inactivity of VKOR linked to decreased bone density and osteoporosis [2].
Inhibition
The most inexpensive and common way to treat blood clotting is through the VKOR inhibitor, . Warfarin is able to do so by outcompeting KO, such that Vitamin K cannot be activated to promote coagulation in the blood. Warfarin will enter the binding pocket of VKOR, creating strong hydrogen bonds with the active site. Warfarin resistance may also occur due to mutations of VKOR, decreasing the effective anticoagulation some drugs may be attempting to promote. The degree of resistance is important to determine so that warfarin may be an effective anticoagulant without being detrimentally effective in blood flow.
Mutations
Some key that can be detrimental to the VKOR structure are mutations of the . The two main residues, N80 and Y139, can be mutated to A80 and F139 creating a decrease in recognition and stabilization.