We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1715
From Proteopedia
(Difference between revisions)
| Line 50: | Line 50: | ||
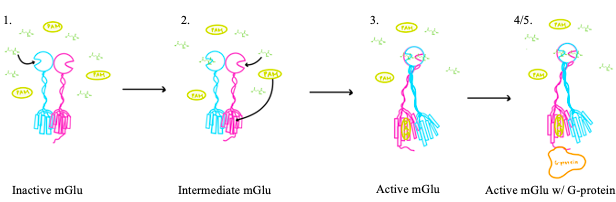
[[Image:Screen Shot 2022-04-15 at 3.54.32 PM.png|300 px|right|thumb|Figure 4. The interaction between an active mGlu and a G-protein ]] | [[Image:Screen Shot 2022-04-15 at 3.54.32 PM.png|300 px|right|thumb|Figure 4. The interaction between an active mGlu and a G-protein ]] | ||
| - | '''3.''' A second glutamate then binds to the other <scene name='90/904320/Active_site_interactions/3'>binding pocket</scene> of the VFT. Mediated by L639, F643, N735, W773, and F776, a <scene name='90/904320/Pam/5'>positive allosteric modulator</scene> (PAM) also binds within the seven TMD helices of the alpha chain <ref name="Seven">PMID:34194039</ref>. This closed conformation of the VFT now has an inter-lobe angle of 25° is considered to be in the <scene name='90/904320/Active_mglu/6'>active conformation</scene><ref name="Seven">PMID:34194039</ref>. The binding of these ligands allows the CRDs to compact and come together. This transformation causes the TMD to form a separate, active asymmetric conformation with a <scene name='90/904319/Active_helices/23'>TM6- | + | '''3.''' A second glutamate then binds to the other <scene name='90/904320/Active_site_interactions/3'>binding pocket</scene> of the VFT. Mediated by L639, F643, N735, W773, and F776, a <scene name='90/904320/Pam/5'>positive allosteric modulator</scene> (PAM) also binds within the seven TMD helices of the alpha chain <ref name="Seven">PMID:34194039</ref>. This closed conformation of the VFT now has an inter-lobe angle of 25° is considered to be in the <scene name='90/904320/Active_mglu/6'>active conformation</scene><ref name="Seven">PMID:34194039</ref>. The binding of these ligands allows the CRDs to compact and come together. This transformation causes the TMD to form a separate, active asymmetric conformation with a <scene name='90/904319/Active_helices/23'>TM6-TM6 interface</scene> between the chains<ref name="Seven">PMID:34194039</ref>. |
'''4.''' The <scene name='90/904320/Active_helices/2'>crossover of the helices</scene> from the alpha and beta chains allows for intracellular loop 2 (ICL2) and the C-terminus to be properly ordered to interact with a G protein<ref name="Seven">PMID:34194039</ref>. While hydrogen bonding is present between the C-terminus and alpha helix 5 of the G-protein, this coupling is primarily driven by the hydrophobic interactions in the interface with the ɑ5 helix of the G protein<ref name="Seven">PMID:34194039</ref>(Figure 4). This <scene name='90/904320/Active_mglu/5'>mGlu/G-protein coupling</scene> can only occur in the presence of a PAM as the pocket in which the coupling occurs would be completely closed in its absence<ref name="Seven">PMID:34194039</ref>. | '''4.''' The <scene name='90/904320/Active_helices/2'>crossover of the helices</scene> from the alpha and beta chains allows for intracellular loop 2 (ICL2) and the C-terminus to be properly ordered to interact with a G protein<ref name="Seven">PMID:34194039</ref>. While hydrogen bonding is present between the C-terminus and alpha helix 5 of the G-protein, this coupling is primarily driven by the hydrophobic interactions in the interface with the ɑ5 helix of the G protein<ref name="Seven">PMID:34194039</ref>(Figure 4). This <scene name='90/904320/Active_mglu/5'>mGlu/G-protein coupling</scene> can only occur in the presence of a PAM as the pocket in which the coupling occurs would be completely closed in its absence<ref name="Seven">PMID:34194039</ref>. | ||
Revision as of 23:26, 17 April 2022
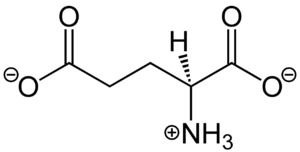
Metabotropic Glutamate Receptor
| |||||||||||
3D Structures
7epa, mGlu Inactive
7mtr, mGlu Active
7mts, mGlu Active G Protein Bound
References
- ↑ Katritch V, Cherezov V, Stevens RC. Structure-function of the G protein-coupled receptor superfamily. Annu Rev Pharmacol Toxicol. 2013;53:531-56. doi:, 10.1146/annurev-pharmtox-032112-135923. Epub 2012 Nov 8. PMID:23140243 doi:http://dx.doi.org/10.1146/annurev-pharmtox-032112-135923
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295-322. doi:, 10.1146/annurev.pharmtox.011008.145533. PMID:20055706 doi:http://dx.doi.org/10.1146/annurev.pharmtox.011008.145533
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 Seven AB, Barros-Alvarez X, de Lapeyriere M, Papasergi-Scott MM, Robertson MJ, Zhang C, Nwokonko RM, Gao Y, Meyerowitz JG, Rocher JP, Schelshorn D, Kobilka BK, Mathiesen JM, Skiniotis G. G-protein activation by a metabotropic glutamate receptor. Nature. 2021 Jun 30. pii: 10.1038/s41586-021-03680-3. doi:, 10.1038/s41586-021-03680-3. PMID:34194039 doi:http://dx.doi.org/10.1038/s41586-021-03680-3
- ↑ 4.0 4.1 4.2 4.3 Lin S, Han S, Cai X, Tan Q, Zhou K, Wang D, Wang X, Du J, Yi C, Chu X, Dai A, Zhou Y, Chen Y, Zhou Y, Liu H, Liu J, Yang D, Wang MW, Zhao Q, Wu B. Structures of Gi-bound metabotropic glutamate receptors mGlu2 and mGlu4. Nature. 2021 Jun;594(7864):583-588. doi: 10.1038/s41586-021-03495-2. Epub 2021, Jun 16. PMID:34135510 doi:http://dx.doi.org/10.1038/s41586-021-03495-2
- ↑ 5.0 5.1 5.2 5.3 5.4 Crupi R, Impellizzeri D, Cuzzocrea S. Role of Metabotropic Glutamate Receptors in Neurological Disorders. Front Mol Neurosci. 2019 Feb 8;12:20. doi: 10.3389/fnmol.2019.00020. eCollection , 2019. PMID:30800054 doi:http://dx.doi.org/10.3389/fnmol.2019.00020
- ↑ Bordi F, Ugolini A. Group I metabotropic glutamate receptors: implications for brain diseases. Prog Neurobiol. 1999 Sep;59(1):55-79. doi: 10.1016/s0301-0082(98)00095-1. PMID:10416961 doi:http://dx.doi.org/10.1016/s0301-0082(98)00095-1
- ↑ Conn PJ, Lindsley CW, Jones CK. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci. 2009 Jan;30(1):25-31. doi: 10.1016/j.tips.2008.10.006. Epub, 2008 Dec 6. PMID:19058862 doi:http://dx.doi.org/10.1016/j.tips.2008.10.006
Student Contributors
- Courtney Vennekotter
- Cade Chezem