We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1705
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
== Introduction == | == Introduction == | ||
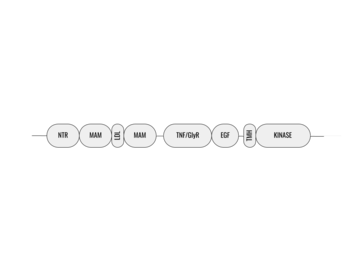
[[Image:ALK_Domain_Outline.png|350 px|right|thumb|Figure 1. Outline of the domains and regions of anaplastic lymphoma kinase]] | [[Image:ALK_Domain_Outline.png|350 px|right|thumb|Figure 1. Outline of the domains and regions of anaplastic lymphoma kinase]] | ||
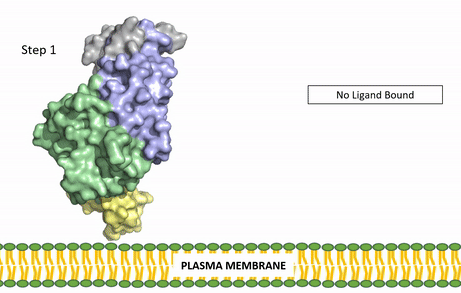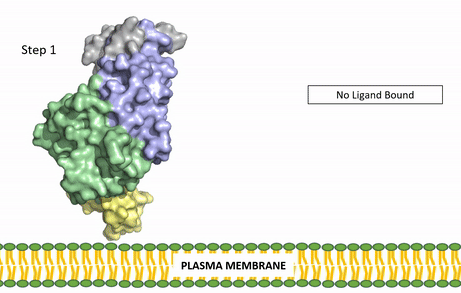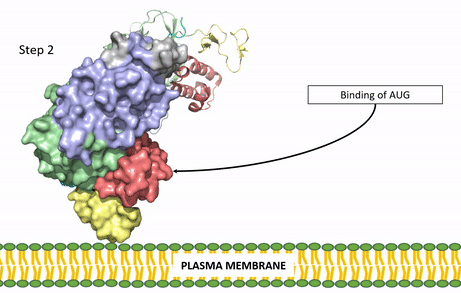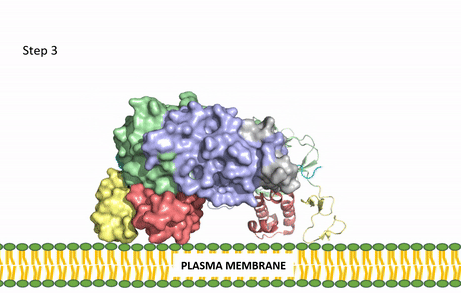
| - | Anaplastic lymphoma kinase is a [https://en.wikipedia.org/wiki/Receptor_tyrosine_kinase receptor tyrosine kinase] (RTK) important to the regulation of functions within the central nervous system <ref name="Reshetnyak">PMID:34819673</ref>. RTKs are the high-affinity cell surface receptors for many polypeptide growth factors, cytokines, and hormones. ALK activates pathways that promote cell growth and proliferation, similar to the Insulin Receptor. These pathways include, but are not limited to, the [https://en.wikipedia.org/wiki/MAPK/ERK_pathway ERK], [https://en.wikipedia.org/wiki/JAK-STAT_signaling_pathway JAK], and [https://en.wikipedia.org/wiki/PI3K/AKT/mTOR_pathway PI3K] pathways. ALK is composed of two identical monomers consisting of seven unique domains and two intermixed regions. One region to note is the glycine-rich region which is highly uncharacteristic in that it has helices composed of only glycine. The preferred ligand for binding is AUG which binds in a dimeric fashion to ALK. When the ligand has bound, there is a conformational change that is covered in more detail in Figure 2 and it's accompanying text. In order to determine the atomic details of human ALK dimerization and activation by AUG, the methods of [https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy cryo-electron microscopy],[https://en.wikipedia.org/wiki/Nuclear_magnetic_resonance_spectroscopy nuclear magnetic resonance], and [https://en.wikipedia.org/wiki/X-ray_crystallography X-ray crystallography] were utilized <ref name="Reshetnyak">PMID:34819673</ref> . Anaplastic lymphoma kinase is a proto-oncogene with mutations associated with various types of cancers, including non-small-cell lung cancer, anaplastic large cell lymphoma, squamous cell carcinoma, and inflammatory myofibroblastic cancer <ref name="Palmer">PMID:19459784</ref>. ALK is a referred to as a proto-oncogene because certain mutations in it's protein sequence are known to have a strong positive association with the development of cancerous cells. | + | Anaplastic lymphoma kinase is a [https://en.wikipedia.org/wiki/Receptor_tyrosine_kinase receptor tyrosine kinase] (RTK) important to the regulation of functions within the central nervous system <ref name="Reshetnyak">PMID:34819673</ref>. RTKs are the high-affinity cell surface receptors for many polypeptide growth factors, cytokines, and hormones. ALK activates pathways that promote cell growth and proliferation, similar to the [[Insulin Receptor|Insulin Receptor (IR)]]. These pathways include, but are not limited to, the [https://en.wikipedia.org/wiki/MAPK/ERK_pathway ERK], [https://en.wikipedia.org/wiki/JAK-STAT_signaling_pathway JAK], and [https://en.wikipedia.org/wiki/PI3K/AKT/mTOR_pathway PI3K] pathways. ALK is composed of two identical monomers consisting of seven unique domains and two intermixed regions. One region to note is the glycine-rich region which is highly uncharacteristic in that it has helices composed of only glycine. The preferred ligand for binding is AUG which binds in a dimeric fashion to ALK. When the ligand has bound, there is a conformational change that is covered in more detail in Figure 2 and it's accompanying text. In order to determine the atomic details of human ALK dimerization and activation by AUG, the methods of [https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy cryo-electron microscopy],[https://en.wikipedia.org/wiki/Nuclear_magnetic_resonance_spectroscopy nuclear magnetic resonance], and [https://en.wikipedia.org/wiki/X-ray_crystallography X-ray crystallography] were utilized <ref name="Reshetnyak">PMID:34819673</ref> . Anaplastic lymphoma kinase is a proto-oncogene with mutations associated with various types of cancers, including non-small-cell lung cancer, anaplastic large cell lymphoma, squamous cell carcinoma, and inflammatory myofibroblastic cancer <ref name="Palmer">PMID:19459784</ref>. ALK is a referred to as a proto-oncogene because certain mutations in it's protein sequence are known to have a strong positive association with the development of cancerous cells. |
Revision as of 14:46, 18 April 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Reshetnyak AV, Rossi P, Myasnikov AG, Sowaileh M, Mohanty J, Nourse A, Miller DJ, Lax I, Schlessinger J, Kalodimos CG. Mechanism for the activation of the anaplastic lymphoma kinase receptor. Nature. 2021 Dec;600(7887):153-157. doi: 10.1038/s41586-021-04140-8. Epub 2021, Nov 24. PMID:34819673 doi:http://dx.doi.org/10.1038/s41586-021-04140-8
- ↑ Palmer RH, Vernersson E, Grabbe C, Hallberg B. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J. 2009 May 27;420(3):345-61. doi: 10.1042/BJ20090387. PMID:19459784 doi:http://dx.doi.org/10.1042/BJ20090387
- ↑ Li T, Stayrook SE, Tsutsui Y, Zhang J, Wang Y, Li H, Proffitt A, Krimmer SG, Ahmed M, Belliveau O, Walker IX, Mudumbi KC, Suzuki Y, Lax I, Alvarado D, Lemmon MA, Schlessinger J, Klein DE. Structural basis for ligand reception by anaplastic lymphoma kinase. Nature. 2021 Dec;600(7887):148-152. doi: 10.1038/s41586-021-04141-7. Epub 2021, Nov 24. PMID:34819665 doi:http://dx.doi.org/10.1038/s41586-021-04141-7
- ↑ Huang H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int J Mol Sci. 2018 Nov 2;19(11). pii: ijms19113448. doi: 10.3390/ijms19113448. PMID:30400214 doi:http://dx.doi.org/10.3390/ijms19113448
- ↑ 5.0 5.1 Selander-Sunnerhagen M, Ullner M, Persson E, Teleman O, Stenflo J, Drakenberg T. How an epidermal growth factor (EGF)-like domain binds calcium. High resolution NMR structure of the calcium form of the NH2-terminal EGF-like domain in coagulation factor X. J Biol Chem. 1992 Sep 25;267(27):19642-9. PMID:1527084
- ↑ 6.0 6.1 Li T, Stayrook SE, Tsutsui Y, Zhang J, Wang Y, Li H, Proffitt A, Krimmer SG, Ahmed M, Belliveau O, Walker IX, Mudumbi KC, Suzuki Y, Lax I, Alvarado D, Lemmon MA, Schlessinger J, Klein DE. Structural basis for ligand reception by anaplastic lymphoma kinase. Nature. 2021 Dec;600(7887):148-152. doi: 10.1038/s41586-021-04141-7. Epub 2021, Nov 24. PMID:34819665 doi:http://dx.doi.org/10.1038/s41586-021-04141-7
- ↑ 7.0 7.1 De Munck S, Provost M, Kurikawa M, Omori I, Mukohyama J, Felix J, Bloch Y, Abdel-Wahab O, Bazan JF, Yoshimi A, Savvides SN. Structural basis of cytokine-mediated activation of ALK family receptors. Nature. 2021 Oct 13. pii: 10.1038/s41586-021-03959-5. doi:, 10.1038/s41586-021-03959-5. PMID:34646012 doi:http://dx.doi.org/10.1038/s41586-021-03959-5
- ↑ Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer. 2013 Oct;13(10):685-700. doi: 10.1038/nrc3580. PMID:24060861 doi:http://dx.doi.org/10.1038/nrc3580
- ↑ Lewis RT, Bode CM, Choquette D, Potashman M, Romero K, Stellwagen JC, Teffera Y, Moore E, Whittington DA, Chen H, Epstein LF, Emkey R, Andrews PS, Yu V, Saffran DC, Xu M, Drew AE, Merkel P, Szilvassy S, Brake RL. The discovery and optimization of a novel class of potent, selective and orally bioavailable Anaplastic Lymphoma Kinase (ALK) Inhibitors with potential utility for the treatment of cancer. J Med Chem. 2012 Jun 26. PMID:22734674 doi:10.1021/jm3005866
- ↑ 10.0 10.1 Sahu A, Prabhash K, Noronha V, Joshi A, Desai S. Crizotinib: A comprehensive review. South Asian J Cancer. 2013 Apr;2(2):91-7. doi: 10.4103/2278-330X.110506. PMID:24455567 doi:http://dx.doi.org/10.4103/2278-330X.110506
- ↑ 11.0 11.1 Wang Q, Zorn JA, Kuriyan J. A structural atlas of kinases inhibited by clinically approved drugs. Methods Enzymol. 2014;548:23-67. doi: 10.1016/B978-0-12-397918-6.00002-1. PMID:25399641 doi:http://dx.doi.org/10.1016/B978-0-12-397918-6.00002-1
PDB Files Used
Student Contributors
- Kaylin Todor
- Rebekah White